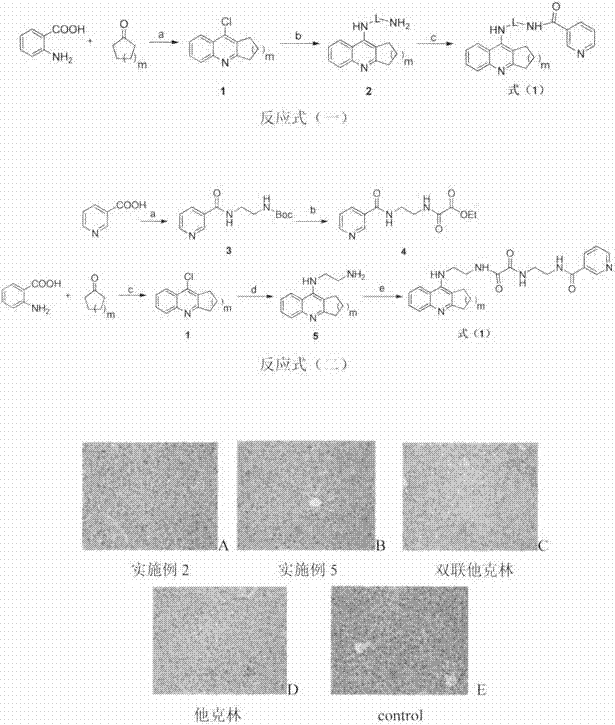
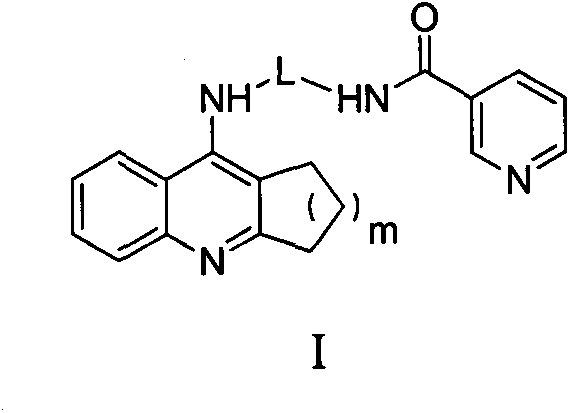
Tacrine- nicotinic acid conjugate, preparation method and medical application thereof
A compound and medicinal salt technology, applied in tacrine-nicotinic acid conjugates, in the application field of Alzheimer's disease treatment, can solve the problems of high liver toxicity, cognitive function defects, and large dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of N-(6-((2,3-dihydro-1H-cyclopenta[b]quinolin-9-yl)amino)hexyl)nicotinamide
[0054] (1) 9-Chloro-2,3-dihydro-1H-cyclopenta[b]quinoline
[0055]
[0056] Anthranilic acid (5.55g, 40.5mmol) and cyclopentanone (4.02ml, 45mmol) were placed in a 100ml three-necked flask, and 33.6ml of POCl was slowly added dropwise under an ice bath 3 , heated to reflux at 105°C for 3h, allowed to cool to room temperature, spin-dried under reduced pressure and vacuum, mixed solvent CH 2 Cl 2 .CH 3 Dissolve in OH (100ml, v:v=2:1), neutralize with saturated potassium carbonate, wash with saturated brine (200ml*5), dry the organic layer over anhydrous sodium sulfate, and separate by column chromatography (PE:EtOAc, v:v= 20:4) to obtain 5.76 g of 9-chloro-2,3-dihydro-1H-cyclopenta[b]quinoline with a yield of 70%. m.p.58-61℃. 1 H NMR (CDCl 3 , 500MHz): 8.17-8.15 (1H, d, J=8.35Hz), 8.05-8.03 (1H, d, J=8.35Hz), 7.69-7.66 (1H, t, J=8.2Hz), 7.58-7.55 ( 1H, t, J=8.05Hz), 3.26-3...
Embodiment 2
[0064] Preparation of N-(6-((1,2,3,4-tetrahydroacridin-9-yl)amino)hexyl)nicotinamide
[0065] (1) 9-chloro-1,2,3,4-tetrahydroacridine
[0066]
[0067] Anthranilic acid (5.55g, 40.5mmol) and cyclohexanone (4.02mL, 45.0mmol) were added to a 100ml three-necked flask, and 33.6ml of POCl was slowly added dropwise under an ice bath 3 , Reflux at 105°C for 3h, and let the reaction solution cool to room temperature. Spin dry under reduced pressure, mixed solvent CH 2 Cl 2 :CH 3Dissolve in OH (100ml, v:v=2:1), neutralize with saturated potassium carbonate, wash with saturated brine (200ml*5), dry the organic layer over anhydrous sodium sulfate, and separate by column chromatography (PE:EtOAc, v:v= 20:4), to obtain 4.8 g of solid 9-chloro-1,2,3,4-tetrahydroacridine. m.p.67-69℃, hydrogen spectrum data consistent with literature reports.
[0068] (2)N 1 -(1,2,3,4-tetrahydroacridin-9-yl)hexane-1,6-diamine
[0069]
[0070] 9-Chloro-1,2,3,4-tetrahydroacridine (2.17g, 1mmol) a...
Embodiment 3
[0075] Preparation of N-(7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptyl)nicotinamide
[0076] (1)N 1 -(1,2,3,4-tetrahydroacridin-9-yl)heptane-1,7-diamine
[0077]
[0078] Reference Example 2, using 9-chloro-1,2,3,4-tetrahydroacridine and 1,7-heptanediamine as raw materials to synthesize viscous compound N 1 -(1,2,3,4-tetrahydroacridin-9-yl)heptane-1,7-diamine, yield: 75%. 1 H NMR (DMSO-d6, 300MHz): 8.20-8.17 (1H, d, J=8.76Hz), 7.69-7.66 (1H, d, J=8.16HZ), 7.54-7.49 (1H, t, J=6.90Hz ), 7.35-7.30(1H, t, J=7.92Hz), 3.51-3.50(2H, m), 3.24-3.23(2H, m), 3.03-3.01(2H, m), 2.89-2.84(2H, m ), 2.76-2.73 (4H, m), 2.09-2.00 (4H, m), 1.52 (6H, br); MS (m / z): 312.2 [M+H] + .
[0079] (2) N-(7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptyl)nicotinamide
[0080]
[0081] Referring to Example 2, with N 1 Synthesis of N-(7-(1,2,3,4-tetrahydro Acridin-9-ylamino)heptyl)nicotinamide, yield 85%. 1 H NMR (CDCl 3 , 500MHz): 9.06-9.05 (d, 1H, J=1.65Hz), 8.68-8.66 (dd, 1H, J 1 = 4.8Hz,J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com