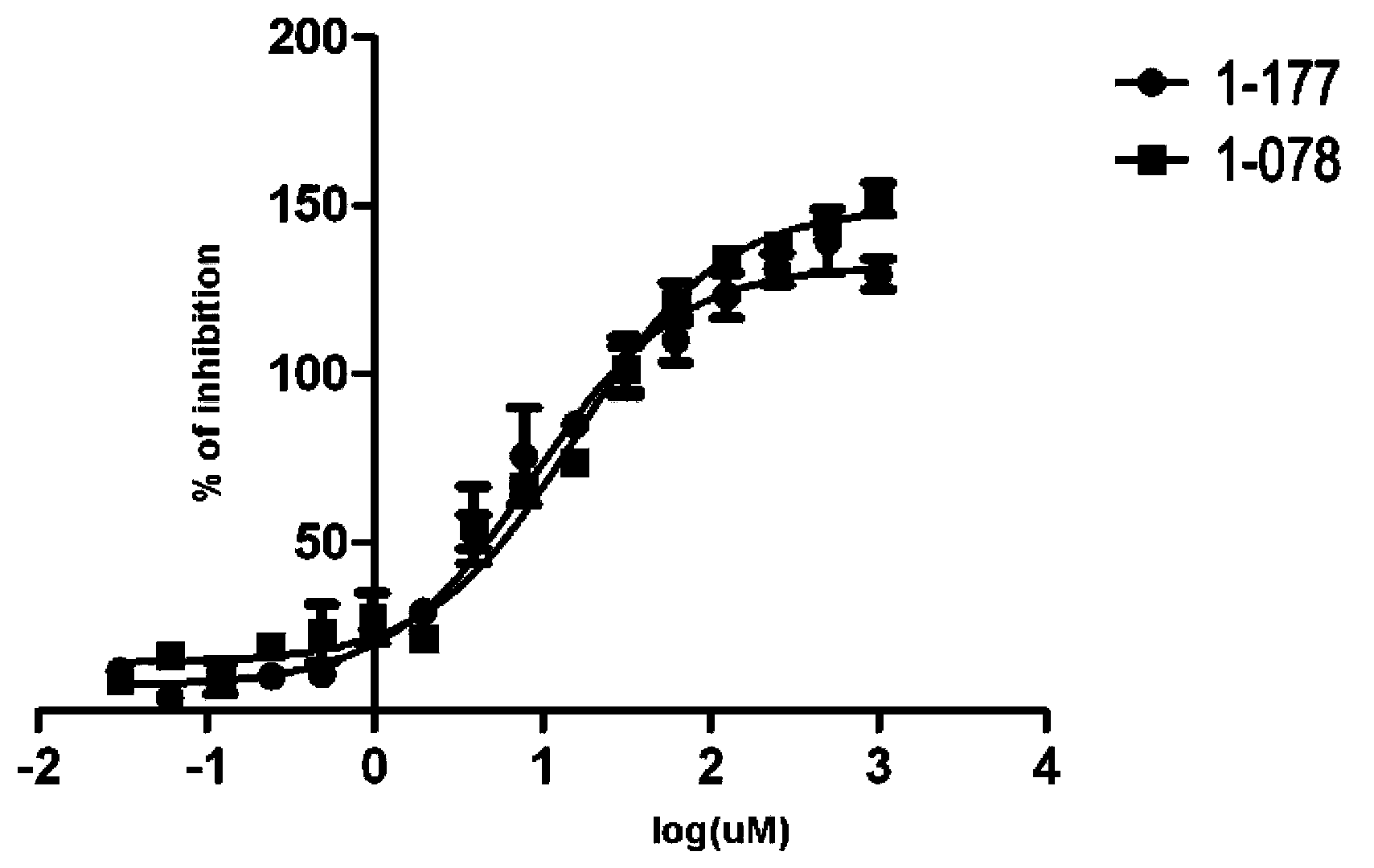
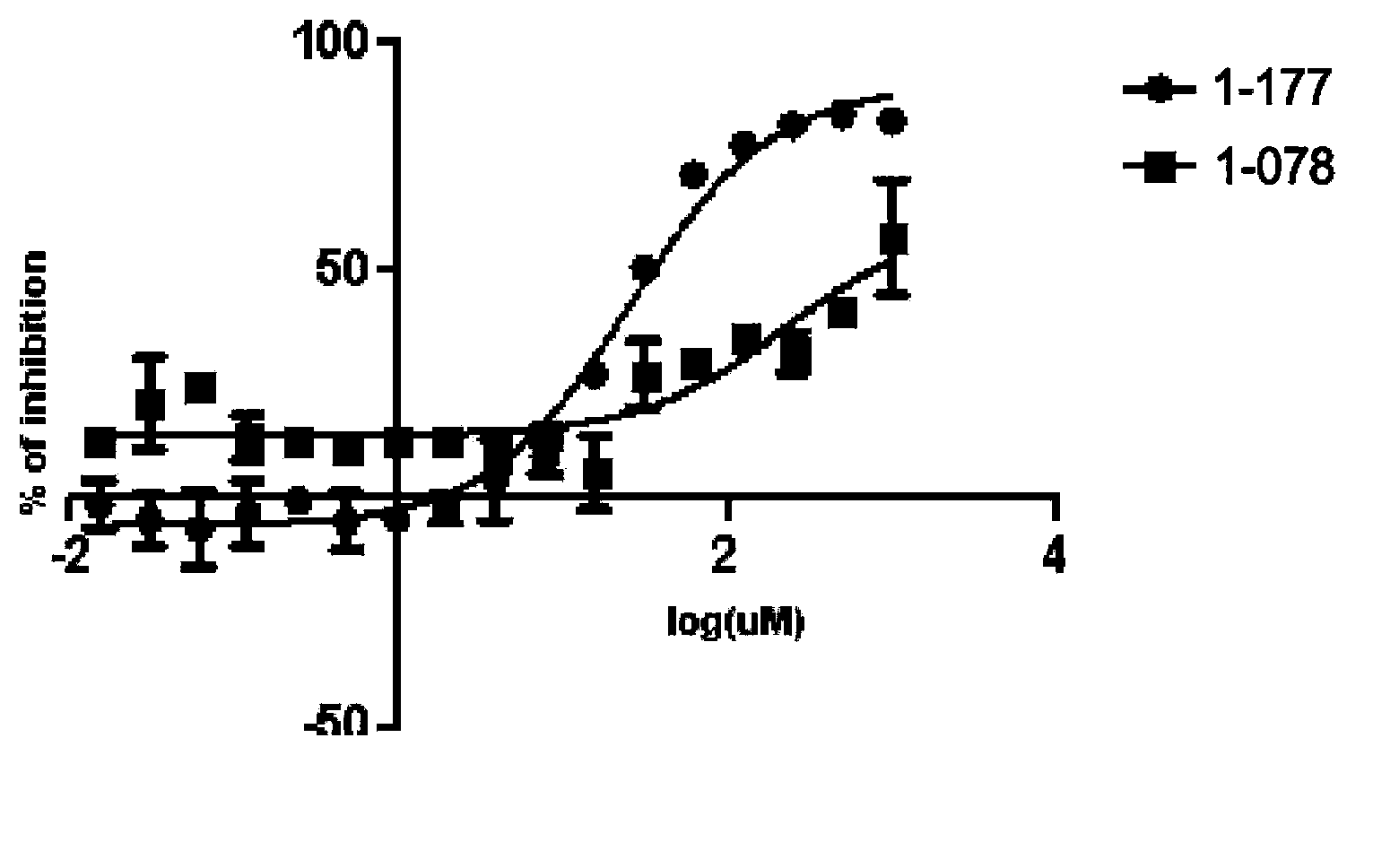
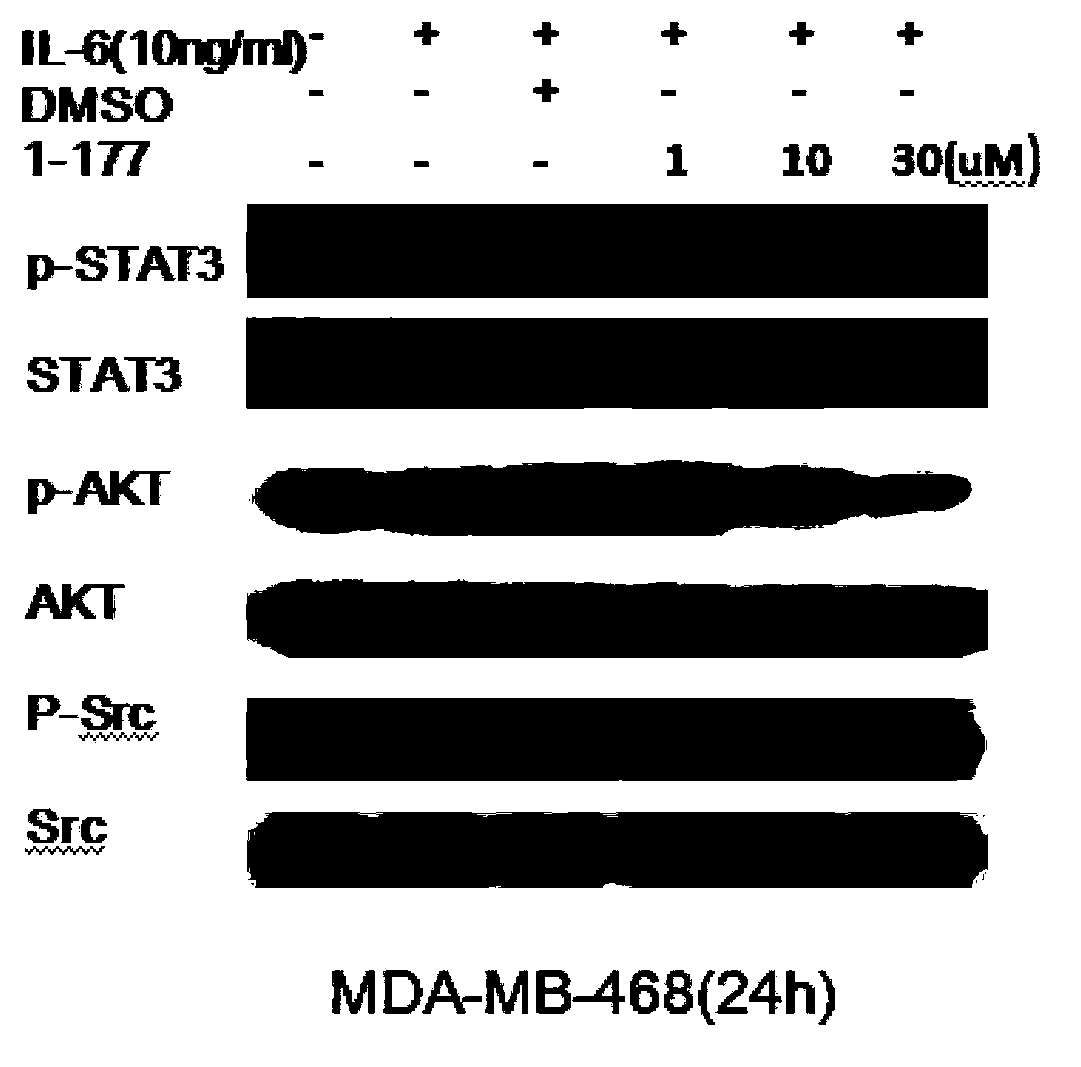
STAT3 small-molecule inhibitor and its application
A small molecule inhibitor and inhibitor technology, applied in the field of tumor drugs, can solve the problems of incomplete inhibition, poor cell permeability, limited inhibitory activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1. Preparation of compound general formula (2a)
[0031]
[0032] General formula (2a) is a specific compound type of general formula (1) and general formula (1a). Since part B can be a ring or chain structure, it is represented by R in general formula (2a).
[0033]
[0034] Substituted or unsubstituted benzene rings, heterocycles, cinnamic acids or aliphatic acids are refluxed in anhydrous ethanol-concentrated sulfuric acid (20:1) solution for 9 hours, spin to dry 2 / 3 of the solvent, and add an appropriate amount of crushed ice to the residue , then NaHCO 3 The pH of the solution was adjusted to neutral, and the product was extracted with ethyl acetate, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain a colorless liquid, namely product 3. Dissolve the ester obtained above in absolute ethanol, add an equivalent amount of hydrazine hydrate, and reflux for 24 hours. Spin off part of...
preparation approach 1
[0042]
preparation approach 2
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com