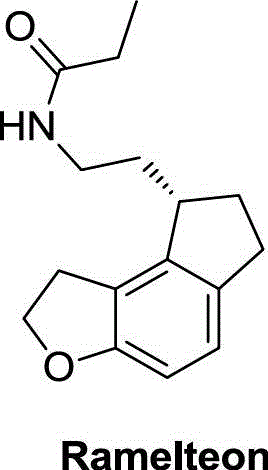
A kind of preparation method of ramelteon key intermediate
A technology of ramelteon and intermediates, which is applied in the field of compound preparation, can solve problems such as inability to obtain easily, and achieves the effects of novel synthetic route, high yield and short synthetic linearity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
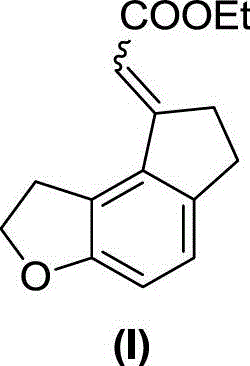
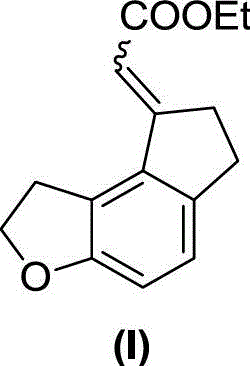
[0033] Example 1. Synthesis of ethyl 2-(6,7-dihydro-1H-indeno[5,4-b]furan-8(2H)-ylidene)acetate (I)
[0034] The chemical reaction formula of embodiment 1 is as follows:
[0035]
[0036] In a dry sealed tube, add formula 1 compound (76g, 367mmol), formula 2 compound (24g, 73.4mmol), triphenylphosphine (3.85g, 14.7mmol), cesium carbonate (143.5g, 440mmol), norbornene ( 34.6g, 367mmol), palladium acetate (1.65g, 7.34mmol), add 240mL dry DME to dissolve, seal the tube at 100°C for 48 hours, complete the reaction, filter, concentrate, dissolve EA (ethyl acetate), wash twice with water , saturated saline, dried over anhydrous sodium sulfate, concentrated, first PE column chromatography, not isolated pure, petroleum ether: ethyl acetate (PE:EA, volume ratio) = 30:1 column chromatography, a pair of cis-trans Isomers: 3.55 g (pale yellow solid) and 11 g (yellow oil), yields of 20% and 61%, respectively.
[0037] Formula (I) compound isomer one: 1 H NMR (400MHz, CDCl 3 )δ7.09(d...
Embodiment 2
[0039] Example 2. Synthesis of ethyl 2-(6,7-dihydro-1H-indeno[5,4-b]furan-8(2H)-ylidene)acetate (I)
[0040] The chemical reaction formula of embodiment 2 is as follows:
[0041]
[0042]In a dry sealed tube, add the compound of formula 1 (1.52g, 7.34mmol), the compound of formula 2 (2.4g, 7.34mmol), triphenylphosphine (385mg, 1.47mmol), cesium carbonate (14.35g, 44mmol), norbornanol After ethylene (3.46g, 36.7mmol) and palladium acetate (165mg, 0.74mmol), add 15mL of dry DME to dissolve, seal the tube at 100°C for 48 hours, complete the reaction, filter, concentrate, wash 2 times with water after EA dissolves, and wash with saturated salt Water, dried over anhydrous sodium sulfate, concentrated, first PE column chromatography, not isolated pure, PE:EA=30:1 column chromatography, obtained a pair of cis-trans isomers: 181mg (light yellow solid) and 540mg (yellow solid) oil), the yields were 10% and 31%, respectively.
Embodiment 3
[0043] Example 3. Synthesis of ethyl 2-(6,7-dihydro-1H-indeno[5,4-b]furan-8(2H)-ylidene)acetate (I)
[0044] The chemical reaction formula of embodiment 3 is as follows:
[0045]
[0046] In a dry sealed tube, add the compound of formula 1 (4.56g, 22mmol), the compound of formula 2 (2.4g, 7.34mmol), triphenylphosphine (385mg, 1.47mmol), cesium carbonate (14.35g, 44mmol), norbornene (3.46g, 36.7mmol), palladium acetate (165mg, 0.74mmol), add 240mL of dry DME to dissolve, seal the tube at 100°C for 48 hours, complete the reaction, filter, concentrate, wash 2 times with water after EA dissolves, and wash with saturated saline , dried over anhydrous sodium sulfate, concentrated, first PE column chromatography, not isolated pure, PE:EA=30:1 column chromatography, to obtain a pair of cis-trans isomers: 287mg (light yellow solid) and 880mg (yellow oily material), the yields were 16% and 49%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com