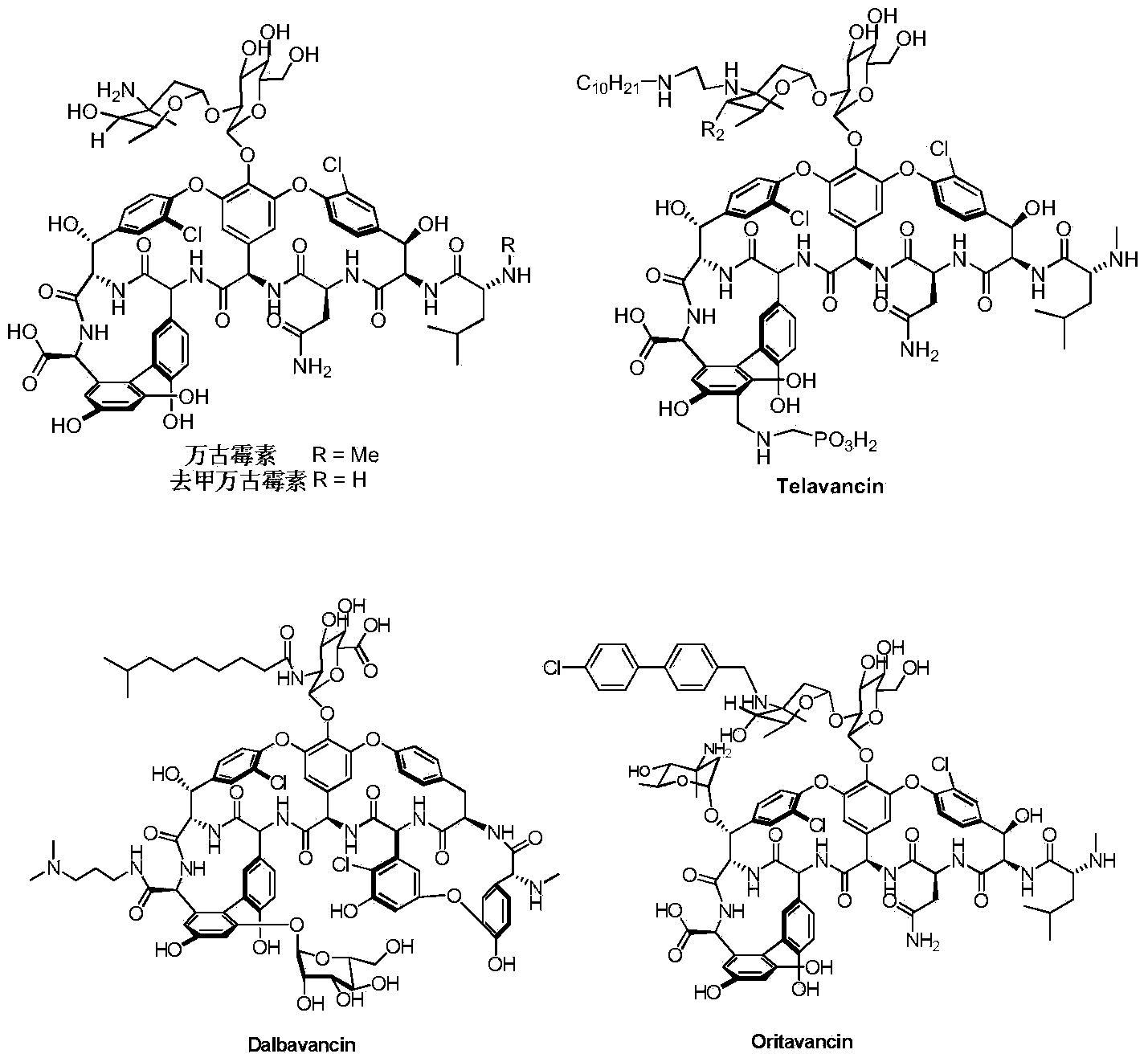
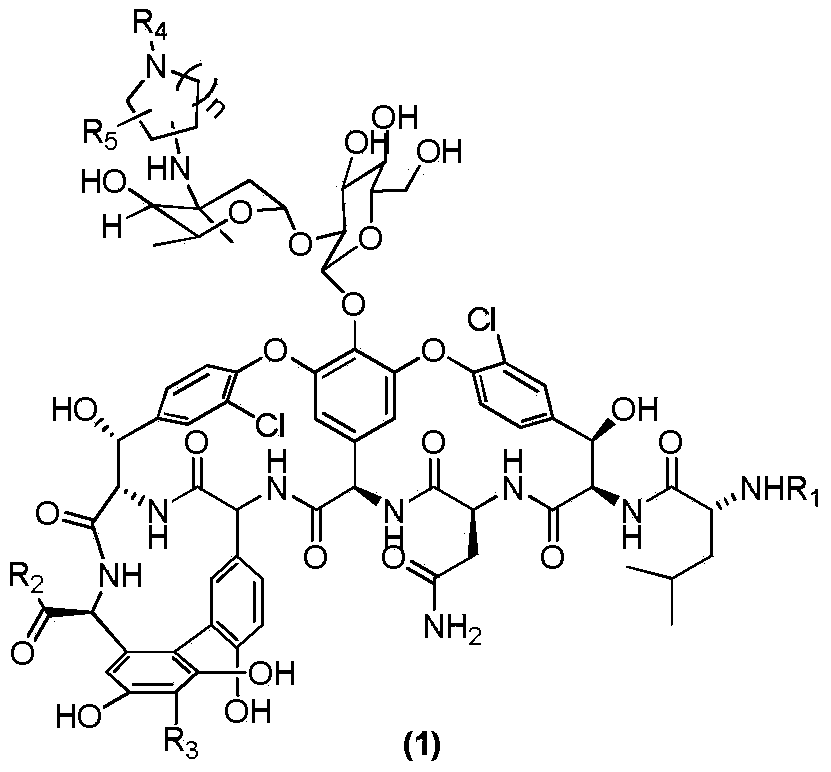
Vancomycin derivative as well as preparation method and pharmaceutical use thereof
A technology of derivatives and antibiotics, applied in the field of medicine, can solve problems such as high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
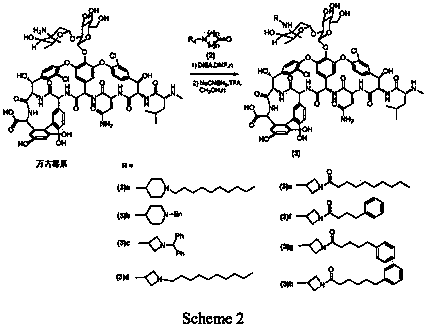
[0057] Synthesis of N-Heterocyclic Amino Substituted Vancomycin
[0058]
[0059] As shown in Scheme2, take a 10ml reaction tube, add vancomycin hydrochloride (80mg, 0.054mmol), dissolve it with 0.8ml dry DMF, add (2) (R 4 =nC 10 H 21 , n=2) 26mg (0.108mmol). DIEA (37μl, 28mg, 0.216mmol) was added dropwise, protected by Ar, and reacted at room temperature for 31h. Then add 320mg (0.324mmol) of NaCNBH, add 0.8ml of methanol to dissolve, add 25μl of TFA (37mg, 0.324mmol) dropwise, and react at room temperature for 15h. The methanol in the reaction solution was distilled off under reduced pressure. 50ml of ether was added to precipitate a white solid. The supernatant was removed. The residue was washed with ether for 30ml*2. After drying, the sample was dissolved with methanol: water=1:4, RP- 18 silica gel column layer, CH3OH:H2O=1:4→1:1 eluted to obtain powdery solid (3)a19mg with a yield of 20%. 1H NMR(DMSO-d6)δ7.53-7.39(m,7H),7.32-7.08(m,9H), 3.82(s,1H), 1.28(s,3H), 1.05(d,J=6....
Embodiment 2
[0064] The final product (3) ah tested for in vitro antibacterial activity against Staphylococcus aureus ATCC25923, Enterococcus faecalis ATCC29212, Methicillin-resistant Staphylococcus aureus 09-250, and Vancomycin-resistant Enterococcus 193,186,435. . Methods: According to the agar double dilution method recommended by CLSI (Clinical Laboratory Standardization Association) in 2006, the minimum inhibitory concentration of antibacterial drugs (Minimal Inhibitory concentration MIC) was determined. Experimental design: Pour 1ml of antibacterial drugs of different types and different concentrations into a 9cm sterile empty plate, and then pour 19ml of sterile MH agar cooled to about 55℃ on the plate immediately, mix well with the drug solution, and cultivate The final concentration of base antibacterial drugs is 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, 0.06 μg / ml; meanwhile, MH plates without antibacterial drugs are prepared as a control. Bacteria inoculation: Add the bacte...
Embodiment 3
[0067] Example 3 In vivo antibacterial protection test
[0068] 1) Test strain
[0069] Clinical isolate: methicillin-resistant Staphylococcus aureus 11002.
[0070] 2) Laboratory animal system:
[0071] Animal grade: clean grade.
[0072] Gender and number: There are 300 KM mice in total, with half male and half female, 150 each.
[0073] Animal weight: 18-22g in weight.
[0074] Source of experimental animals: Shanghai Slack Laboratory Animal Center, Chinese Academy of Sciences, license number SCXK (Shanghai) 2011-0005.
[0075] Animal feeding and management: SPF environmental animal room, laboratory animal use license number: SYXK (Shanghai) 2009-0068.
[0076] Animal feeding: All animals are fed standard sterilized full-price rat feed. Animal drinking water is supplied from drinking bottles. Animals drink freely. Animal feeding: 10 animals per cage before modeling, 5 animals per cage after modeling. The animal is set at room temperature of 20℃~22℃, humidity of 40%~70%, light and dark ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com