Polymer and its preparation method and application
A polymer and group technology, applied in the field of its preparation and polymer, can solve the problems of poor stability of polyethylene glycol, and achieve the effects of excellent biological inertness, wide application range and widening application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The embodiment of the present invention further provides the above polymer preparation method, comprising the following steps:
[0044] Step S01, preparing an intermediate product:
[0045] Add 2-methyl-2-oxazoline and an initiator into an organic solvent, and react at a temperature of 60-80°C for 6-48 hours;
[0046] Step S02, introducing end groups, purifying:
[0047] Adjust the temperature to room temperature, add a terminator, stir and react for 2-24 hours, remove the solvent in the solution after the reaction, add water and stir, and dialyze and dry to obtain a polymer. The initiator is selected from methyl trifluoromethanesulfonate, Iodomethane, ethyl iodide, 1-iodo-n-propane, 1-iodo-isopropane, 1-iodo-n-butane, 1-iodo-tert-isobutane, 1-iodo-tert-butane, etc., the terminator is selected from terminal bands Compounds with hydroxy, amino, mercapto, azido or trimethyl(ethyl)oxyalkyl;
[0048] or,
[0049] Adjust the temperature to room temperature, add a compoun...
Embodiment 1
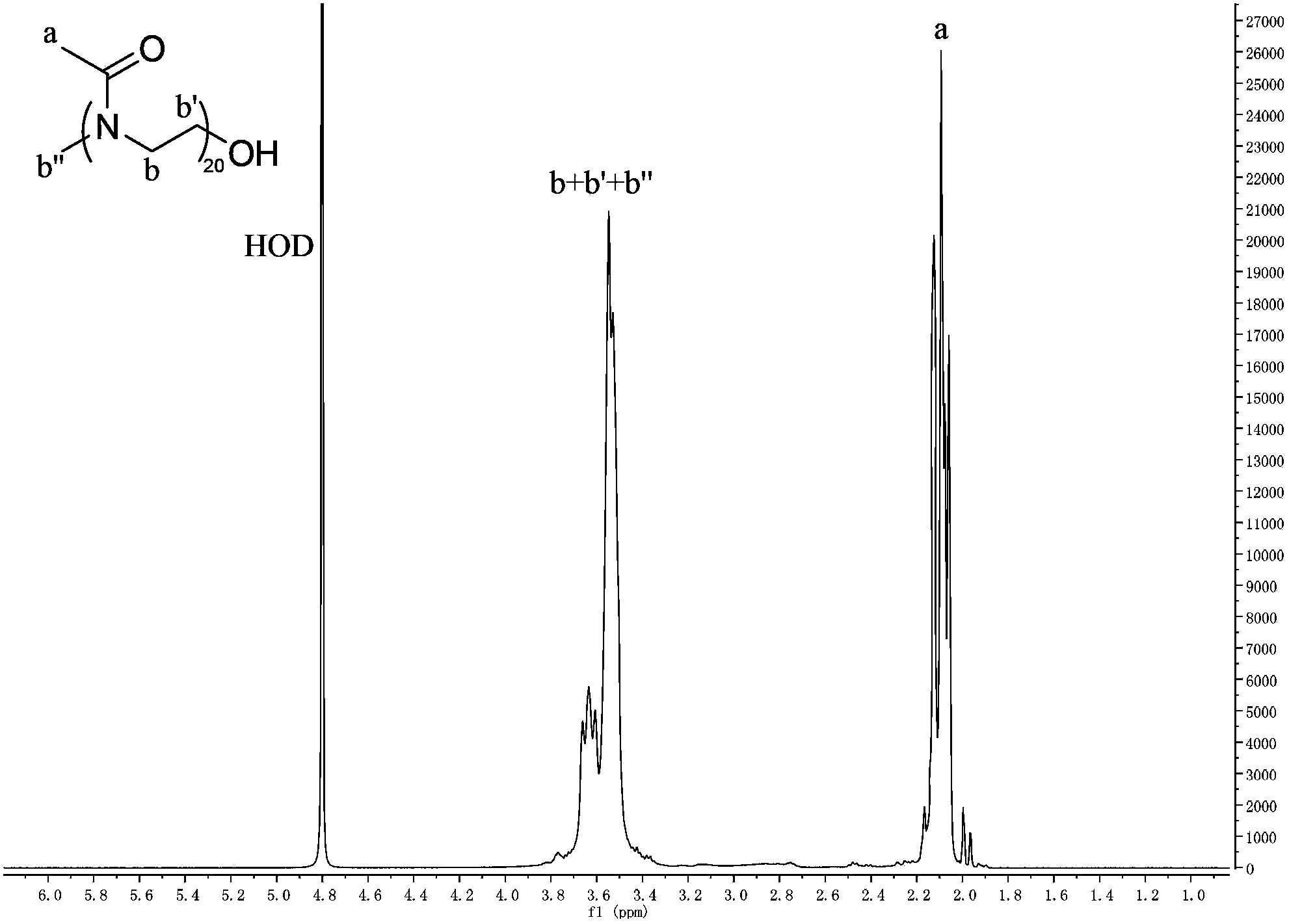
[0070] The chemical structural formula of the embodiment polymer of the present invention is as follows:
[0071]
[0072] The polymer preparation method of the embodiment of the present invention comprises the following steps:
[0073] 2-Methyl-2-oxazoline (8.50mL, 100mmol) was dissolved in acetonitrile (30mL), and methyl trifluoromethanesulfonate (0.44mL, 4.0mmol) was added to the reaction system, after which the mixture was heated to 70°C, react for 8 hours;
[0074] Then stop heating, wait for the mixture to cool to room temperature, and remove part of the solvent by heating under negative pressure to obtain an oily viscous liquid. Adjust to neutrality, then transfer the polymer-containing solution to a dialysis bag, and dialyze in deionized water for 24 hours. Finally, the solution containing the polymer was freeze-dried or dried under reduced pressure to obtain a white solid of poly(2-methyl-2-oxazoline) with an average molecular weight of about 1.8K.
[0075] see ...
Embodiment 2
[0077] The chemical structural formula of the embodiment polymer of the present invention is as follows:
[0078]
[0079] The polymer preparation method of the embodiment of the present invention comprises the following steps:
[0080] 2-Methyl-2-oxazoline (8.50mL, 100mmol) was dissolved in acetonitrile (30mL), then methyl trifluoromethanesulfonate (0.22mL, 2.0mmol) was added to the reaction system, and the mixture was heated to 70°C, react for 16 hours;
[0081] Then the heating was stopped, and when the mixture was cooled to room temperature, ethyl 4-piperidinecarboxylate (2.448 mL, 16 mmol) was added and stirred for 12 hours;
[0082] Part of the solvent was removed by heating under negative pressure to obtain an oily viscous liquid. After adding deionized water to 50mL, it was hydrolyzed for 24 hours under the condition of pH=14, and the pH of the solution was adjusted to neutral. The solution was transferred to a dialysis bag and dialyzed against deionized water for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com