Seminal plasma composite quality control product and its preparation method and kit
A quality control product and kit technology, which is applied in the preparation of test samples, biological testing, material inspection products, etc., can solve the problems of increasing the technical difficulty of seminal plasma quality control products, difficult to store, and unstable, and achieves a high level of avoidance. Unstable, avoids hard-to-save, stable performance effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation method of above-mentioned seminal plasma composite quality control product is:
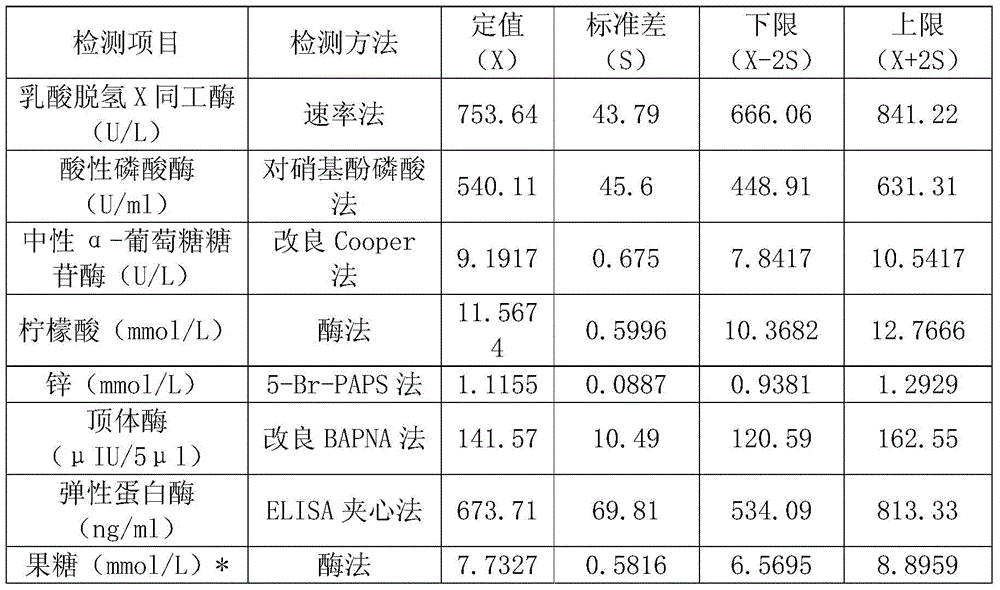
[0024] Preparation of quality control preservation solution: Weigh the above-mentioned sugars, salts and enzyme stabilizers of a certain weight, mix them, add purified water to 100ml, fully dissolve, and obtain a quality control preservation solution containing the above-mentioned percentages of each component.
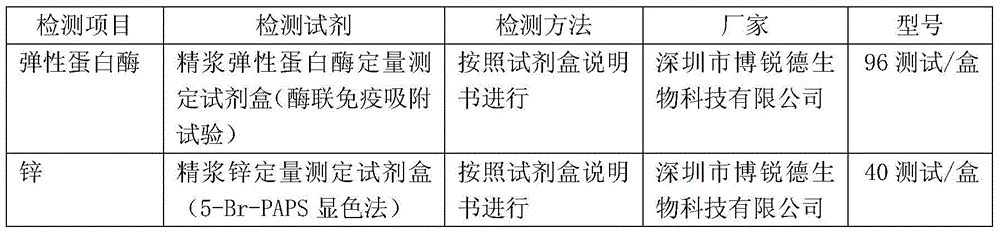
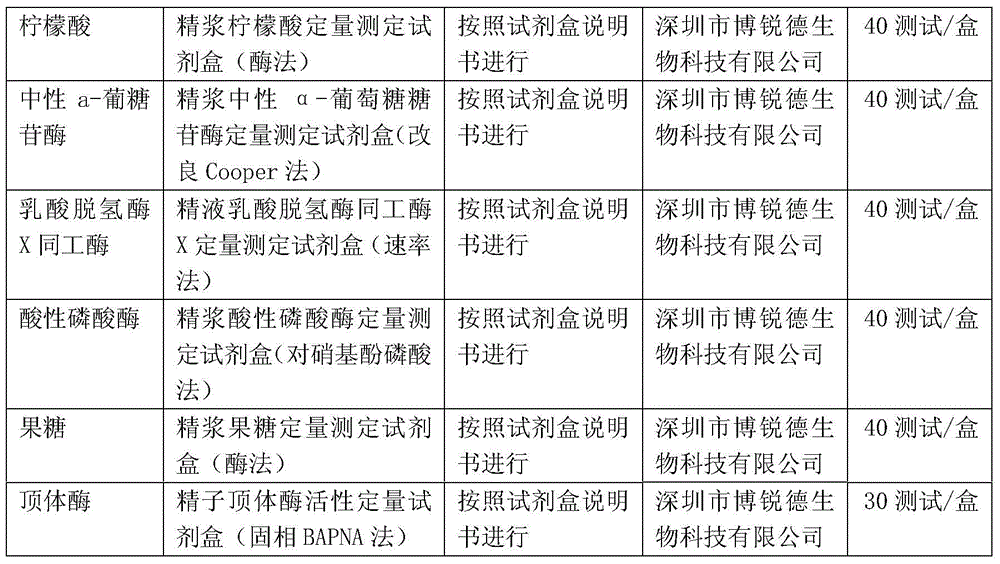
[0025] Obtain seminal plasma stock solution: take several parts of fresh human semen that have been completely liquefied, select HCV, HIV1+2, HBSAg and syphilis negative as raw materials, and centrifuge to obtain seminal plasma (you can also use frozen semen or semen Plasma stock solution is used as raw material, and HCV, HIV1+2, HBSAg and syphilis also need to be detected), and the collected seminal plasma stock solution is mixed by physical methods to obtain seminal plasma stock solution.
[0026] Mix and freeze-dry to obtain seminal plasma composite quality contr...
Embodiment 1
[0037] This embodiment provides a seminal plasma composite quality control product, including: a quality control product preservation solution and a seminal plasma stock solution; the quality control product preservation solution is an aqueous solution containing glycine, sodium chloride and sucrose; wherein, the The quality control product preservation solution and the seminal plasma stock solution were mixed at a volume ratio of 1:1, and then freeze-dried to form a dry powder.
[0038] This embodiment also provides a method for preparing the above-mentioned seminal plasma composite quality control product, the steps of which are as follows:
[0039] Step 1: Prepare quality control preservation solution
[0040] Weigh 0.7302g of aminoacetic acid, 0.1205g of sodium chloride and 0.5713g of sucrose, then add purified water to 100ml, fully dissolve, and obtain a quality control preservation solution.
[0041] Step 2: Obtain seminal plasma stock solution
[0042] Take several pa...
Embodiment 2
[0046] The difference between this embodiment and embodiment 1 is:
[0047] The quality control preservation solution is an aqueous solution containing polyvinylamine, sodium carbonate and glucose; wherein, the quality control preservation solution and the seminal plasma stock solution are mixed at a volume ratio of 1:80 and then freeze-dried to form a dry powder.
[0048] The preparation method of the quality control preservation solution is as follows: weigh 0.8134g of polyvinylamine, 0.0954g of sodium carbonate and 0.6543g of glucose, then add purified water to 100ml, fully dissolve, and obtain the quality control preservation solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com