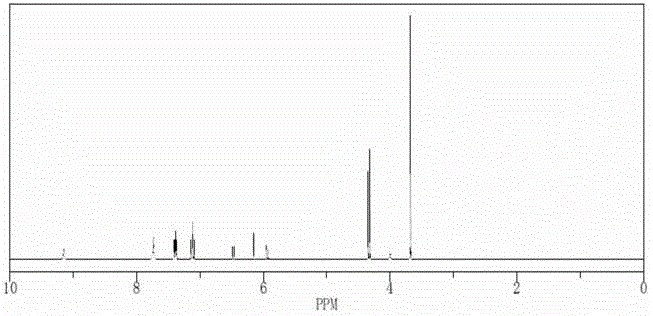
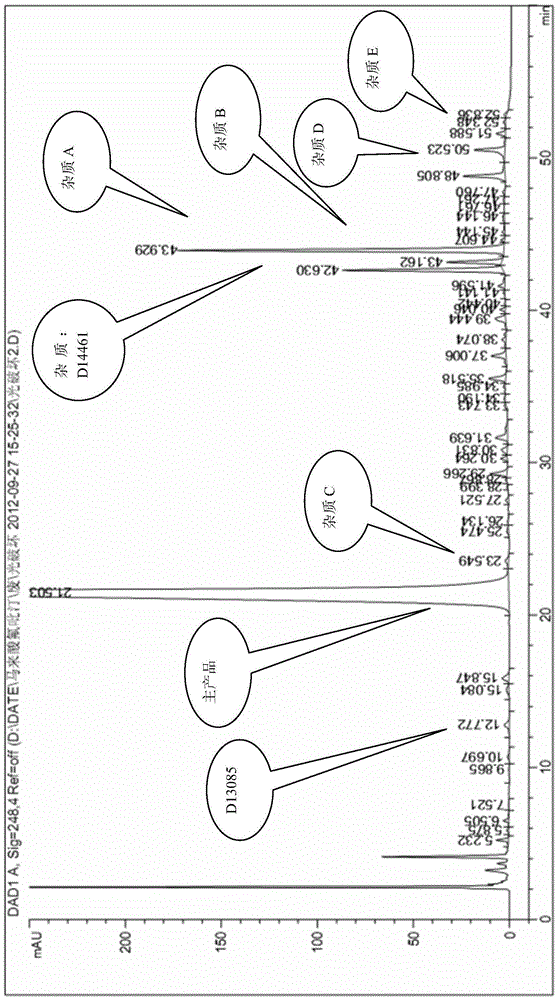
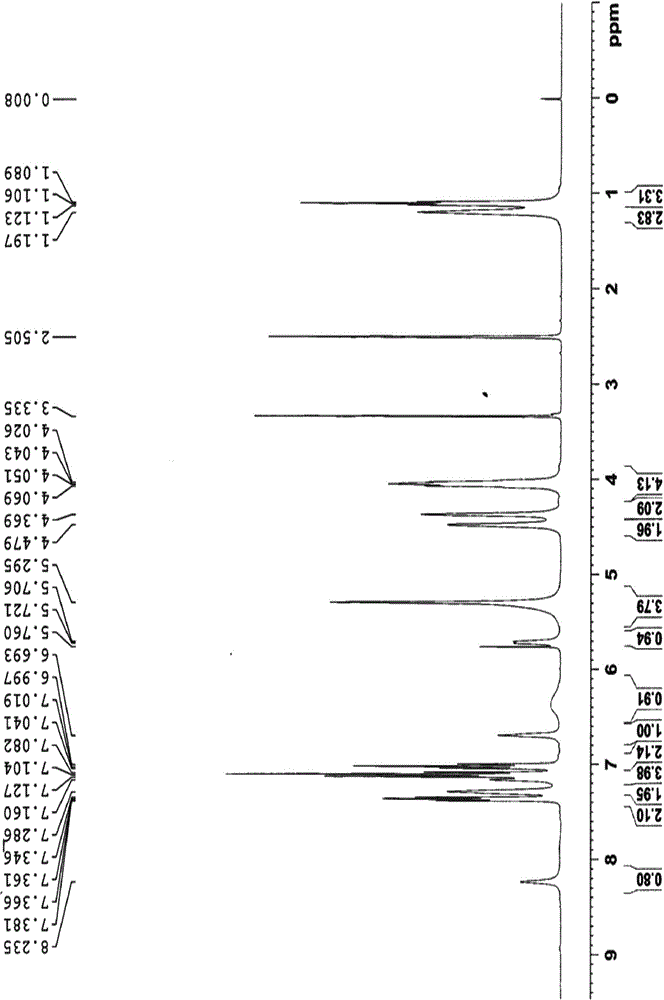
Reference compound used in the analysis of flupirtine maleate
A technology of flupirtine maleate and a compound is applied in the application field of flupirtine maleate drug analysis, which can solve the problems of affecting the accuracy of samples, easy discoloration, instability of flupirtine maleate, etc. High accuracy, good reproducibility, and the effect of improving purity and content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Weigh 20.0 g of flupirtine maleate, add 200 mL of anhydrous methanol, heat to 80°C, stir to dissolve, and keep the temperature for 48 hours. Filtration, column chromatography, eluting with dichloromethane:methanol 100:1 respectively, to obtain impurity A and impurity B.
Embodiment 2
[0082] Dissolve 20g ANFP (2-amino-3-nitro-6-p-fluorobenzylaminopyridine) in anhydrous methanol, add 5% palladium carbon, complete the reaction under 20 kg pressure, spin dry, dissolve in dichloromethane 13 mL of triethylamine was added, methyl chloroformate was added at 0-25°C, and compound C was obtained by spin-drying.
Embodiment 3
[0084] Weigh 20.0 g of compound C, add 200 mL of dimethylformamide: water (20:1), heat to 110°C, stir to dissolve, and keep the temperature for 48 hours. Filtration, column chromatography, eluting with dichloromethane:methanol 100:1 respectively, to obtain impurity D and impurity E.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com