Conjugated polymer containing thienothiophene-dibenzothiophene-benzodithiophene and its preparation method and application
A technology of dibenzothiophene and benzodithiophene is applied in the field of conjugated polymer containing thienothiophene-dibenzothiophenebenzodithiophene and its preparation, and can solve the problems of narrow light absorption range and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
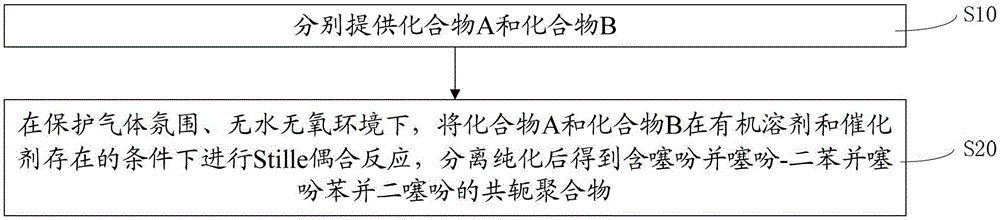
[0061] Such as figure 1 As shown, the preparation method of the above-mentioned thienothiophene-dibenzothiophene benzodithiophene-containing conjugated polymer includes the following steps:
[0062] S10. Compound A and Compound B provided separately.
[0063] Compound A and Compound B have the following structural formulas:
[0064]
[0065] R 1 And R 2 The same is H, C 1 ~C 10 Alkyl or C 1 ~C 10 The alkoxy group, R 3 Is C 1 ~C 16 Alkyl or C 1 ~C 16 的alkoxy。
[0066] The preparation method of compound A includes the following steps:
[0067] S111. Provide compound C and compound E with the following structural formulas:
[0068]
[0069] R 1 And R 2 The same is H, C 1 ~C 10 Alkyl or C 1 ~C 10 的alkoxy。
[0070] S112. Add n-butyllithium to the organic solution of compound C under the conditions of protective gas atmosphere and -78℃, stir and react for 2h~3h, then add trimethyltin chloride to the above system, and react for 0.5h~1h Then return to room temperature and continue to react for 2...
Embodiment 1
[0142] This embodiment discloses a thienothiophene-dibenzothiophene benzodithiophene-containing conjugated polymer P1 with the following structural formula:
[0143]
[0144] Wherein, R is 2-hexyloctyl; n=60.
[0145] The preparation process of conjugated polymer P1 is as follows:
[0146] 1. The monomer compound 4,11-dibromo-7,8-didecyldibenzo[b,b']thiophenebenzo[2,1-b:3,4-b'] with the following structural formula Synthesis of thiophene (A):
[0147]
[0148] 1. Synthesis of the compound 4,5-didecyl-2,7-trimethyltin benzo[2,1-b:3,4-b’]dithiophene (D)
[0149]
[0150] Under the protection of nitrogen, add 2.36g (5mmol) of 4,5-didecylbenzo[2,1-b:3,4-b']dithiophene (C) to 60mL of anhydrous ether solution and cool To -78°C, slowly add 5mL (12mmol) of n-butyllithium in n-hexane solution (2.5M), after the addition, stir the reaction at -78°C for 2h, then add 3.6mL (12mmol) of trimethyl chloride Tin, after holding the reaction for 0.5h, return to room temperature, and continue the reactio...
Embodiment 2
[0188] This embodiment discloses a thienothiophene-dibenzothiophene benzodithiophene-containing conjugated polymer P2 with the following structural formula:
[0189]
[0190] Wherein, n=50; R is 2-ethylhexyl.
[0191] The preparation process of conjugated polymer P2 is as follows:
[0192] 1. The monomer compound 4,11-dibromo-7,8-bis(2-ethylpentyl)dibenzo[b,b']thiophenebenzo[2,1-b:3, Synthesis of 4-b']dithiophene (A):
[0193]
[0194] 1. The compound 4,5-bis(2-ethylpentyl)-2,7-bis(trimethyltin)benzo[2,1-b:3,4-b']dithiophene (D) synthesis
[0195]
[0196] Under the protection of nitrogen, add 1.93g (5mmol) 4,5-bis(2-ethylpentyl)benzo[2,1-b:3,4-b']dithiophene (C) to 60mL dry In the tetrahydrofuran solution, cool to -78°C, slowly add 5mL n-butyllithium (12.5mmol) in n-hexane solution (2.5M), after the addition, stir the reaction at -78°C for 2.5h, and then add 3.8mL of three Methyl tin chloride (12.5mmol), after incubation reaction for 0.6h, return to room temperature, continue the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com