Oxcarbazepine controlled-release tablet and preparation method thereof
A controlled-release tablet and controlled-release material technology, which is applied in pharmaceutical formulations, drug delivery, nervous system diseases, etc., can solve problems such as inability to provide therapeutically effective concentrations and decreased drug bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
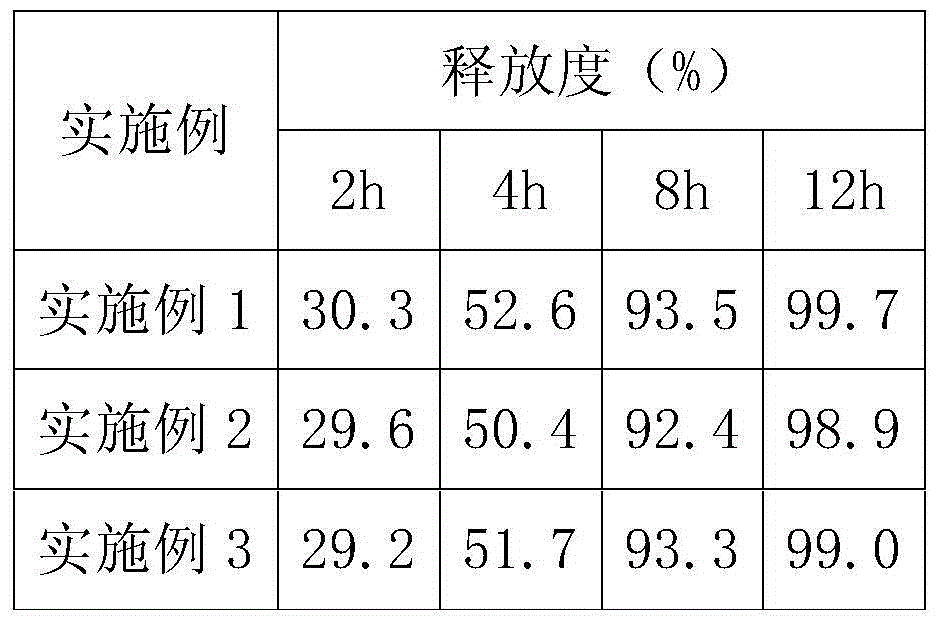
Embodiment 1
[0035] prescription:
[0036] Oxcarbazepine 300g
[0037] Hypromellose 200g
[0038] Microcrystalline Cellulose 100g
[0039] Low-substituted hydroxypropyl cellulose 50g
[0040] Povidone 50g
[0042] Proper amount of ethanol
[0043] Made in 1000 pieces.
[0044] Preparation process:
[0045] 1. Preparation of adhesive: Dissolve the prescribed amount of povidone in ethanol to make a 10% (w / w) adhesive for later use.
[0046] 2. Granulation: After sieving oxcarbazepine and hypromellose with 80 meshes respectively, put them into a mixing granulator together with hypromellose and microcrystalline cellulose, mix them, and add a binder after they are uniform. Wet granulation, take out the granules and use a 20-mesh sieve for wet granulation, after drying, pass through a 20-mesh sieve for granulation, and set aside.
[0047] 3. Tablet compression: After mixing the prepared granules with the prescribed amount of magnesium stearate evenly, test...
Embodiment 2
[0049] Plain Tablet Prescription:
[0050] Oxcarbazepine 300g
[0051] Hypromellose 100g
[0052] Microcrystalline Cellulose 100g
[0053] Mannitol 100g
[0054] Povidone 30g
[0056] Proper amount of ethanol
[0057] Made in 1000 pieces.
[0058] Preparation Process:
[0059] 1. Preparation of adhesive: Dissolve the povidone of the prescribed amount in ethanol to make a 10% (w / w) adhesive for subsequent use;
[0060] 2. Granulation: After sieving oxcarbazepine and mannitol with 80 meshes respectively, put them into the mixing granulator together with hypromellose and microcrystalline cellulose, mix them, and then add binders for wet granulation , Take out the granules and carry out wet sizing with a 20 mesh sieve. After drying, pass through a 20-mesh sieve for granulation and set aside.
[0061] 3. Tablet compression. After mixing the prepared granules with the prescribed amount of magnesium stearate evenly, test the content to determi...
Embodiment 3
[0063] prescription:
[0064] Oxcarbazepine 300g
[0065] Ethylcellulose 150g
[0066] Microcrystalline Cellulose 120g
[0067] Lactose 80g
[0068] Povidone 40g
[0070] Proper amount of ethanol
[0071] Made in 1000 pieces.
[0072] Preparation Process
[0073] 1. Preparation of adhesive: Dissolve the povidone of the prescribed amount in ethanol to make a 10% (w / w) adhesive for subsequent use;
[0074] 2. Granulation. After sieving oxcarbazepine and lactose with 80 meshes respectively, put them into the mixing granulator together with ethyl cellulose and microcrystalline cellulose, mix them, and add binders after they are uniform for wet granulation. Take out The granules are wet sized with a 20-mesh sieve, and after drying, pass through a 20-mesh sieve for granulation and set aside.
[0075] 3. Tablet compression After mixing the prepared granules with the prescribed amount of magnesium stearate evenly, test the content to determine t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com