Preparation method of rifapentine
A technology of rifapentine and rifaxazine, which is applied in the field of preparation of rifapentine, can solve the problems of reducing yield and increasing impurities, and achieves the effects of high impurity content, good stability, and uniform crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be further described in detail through specific implementation examples below.
[0020] The embodiment of the present invention provides a preparation method of rifapentine, comprising the following steps:
[0021] A. Taking rifamycin S as the starting material, reacting with dimethylol terbumine in the first organic solvent and converting it into intermediate rifaxazine;
[0022] B. In the second organic solvent, adding a catalyst and a reducing agent to hydrolyze and open the intermediate rifaxazine;
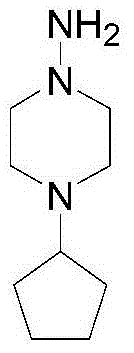
[0023] C. The hydrolyzate of rifaoxazine is condensed into rifapentine solution with 1-amino-4-cyclopentylpiperazine;
[0024] D. Filtration of the rifapentine solution, segmental crystallization of multi-stage crystallization temperature, first separation, washing, second separation, drying, soaking, third separation, rinsing, spin-drying, sieving, drying , Mixing and refining process to get Rifapentine finished product.
[0025] Using rif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com