Medicament of 1,3,4-oxadiazole containing pyrazole compound prepared for treating tumor
A compound, oxadiazole technology, applied in the field of medicinal chemistry, can solve the problem of urgency in the development of anti-tumor drugs, and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
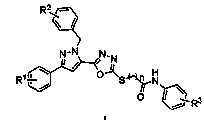
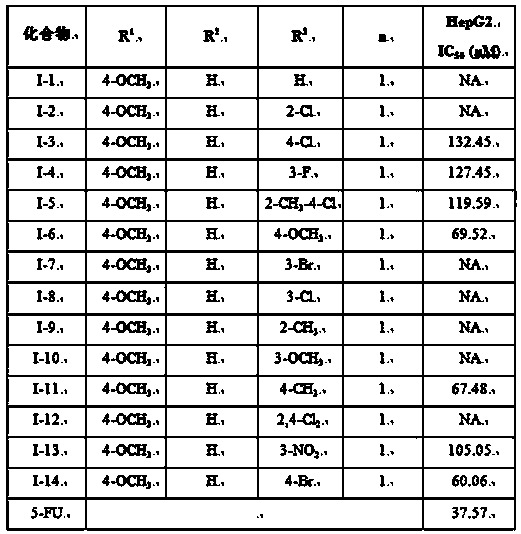
[0037] 5-[3-(4-Methoxyphenyl)-1-arylmethylpyrazol-5-yl]-2-(N-phenylacetamide-2-sulfanyl)-1,3,4- Preparation of oxadiazole (I-1)
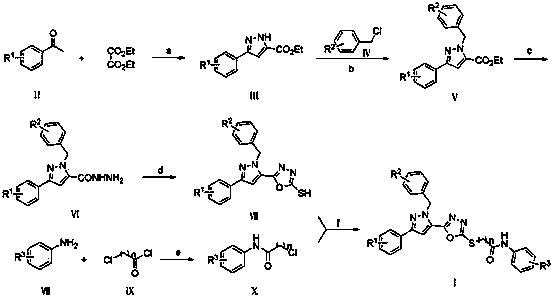
[0038]Under an ice-water bath, slowly add 2.07 g (0.09 mol) of sodium to 50 mL of ethanol. After it is completely dissolved, add dropwise a mixed solution of 10.35 mL (0.075 mol) of ethyl oxalate and 30 mL of ethanol at room temperature, and stir evenly. Add 12.69 mL (0.093 mol) of 4-methoxyacetophenone (II-1) dropwise, and drop it for 1.5 h. After stirring for 4 h, filter under reduced pressure, wash with ethanol three times, and dry in vacuo to obtain a light yellow solid Compound 16.32 g.
[0039] Dissolve 1.09 g (4.5 mmol) of the light yellow solid compound in 10 mL of acetic acid, add 0.35 mL (5.6 mmol) of 80% hydrazine hydrate, N 2 Reflux for 4 h under protection, cool to room temperature, pour the reaction solution into an ice-water mixture, precipitate a light yellow solid under stirring, filter under reduced pressure, wash the filter cake...
Embodiment 2
[0052] 5-[3-(4-methoxyphenyl)-1-arylmethylpyrazol-5-yl]-2-(N-(2-chlorophenyl)acetamide-2-thio)-1 , Preparation of 3,4-oxadiazole (I-2)
[0053] Replace aniline (VIII-1) with 2-chloroaniline (VIII-2), follow the method described in Example 1, and all the other required raw materials and reagents are the same as Example 1 to obtain a yellow-white solid 5-[3-(4- Methoxyphenyl)-1-arylmethylpyrazol-5-yl]-2-(N-(2-chlorophenyl)acetamide-2-thio)-1,3,4-oxadiazole , yield 87%.
[0054] Mp: 212-213 °C; 1 H NMR (DMSO- d6 ) δ: 3.77 (s, 3H, OCH 3 ), 4.23~4.37 (m, 2H, SCH 2 CO), 5.70 (s, 2H, CH 2 Ph), 6.98 (t, J = 8.5 Hz, 2H, ArH), 7.17~7.32 (m, 6H, ArH), 7.53 (s, 3H, ArH), 7.67 (d, J = 8.3 Hz, 3H, ArH), 11.04 (s, 1H, NH); ESI-MS m / z: 532.2 [M+H] + , 554.2 [M+Na] + ; IR (KBr, v ): 3433, 3175, 3153, 3064, 2934, 2843, 2359, 2041, 1731, 1654, 1626, 1543, 1515, 1495, 1480, 1449, 1438, 1417, 1368, 13693, 180, 13 1127, 1110, 1064, 1026 cm -1 .
Embodiment 3
[0056] 5-[3-(4-methoxyphenyl)-1-arylmethylpyrazol-5-yl]-2-(N-(4-chlorophenyl)acetamide-2-thio)-1 , Preparation of 3,4-oxadiazole (I-3)
[0057] With 4-chloroaniline (VIII-3) instead of aniline (VIII-1), according to the method described in Example 1, all the other required raw materials and reagents are the same as in Example 1 to obtain a yellow solid 5-[3-(4-methyl Oxyphenyl)-1-arylmethylpyrazol-5-yl]-2-(N-(4-chlorophenyl)acetamide-2-thio)-1,3,4-oxadiazole, Yield 87%.
[0058] Mp: 239-241 °C; 1 H NMR (DMSO- d6 ) δ: 3.78 (s, 3H, OCH 3 ), 4.19 (s, 2H, SCH 2 CO), 5.69 (s, 2H, CH 2 Ph), 6.98 (t, J = 8.6 Hz, 2H, ArH), 7.18~7.33 (m, 6H, ArH), 7.40 (d, J = 8.5 Hz, 2H, ArH), 7.59 (d, J = 8.5 Hz, 2H, ArH), 7.68 (d, J = 8.4 Hz, 2H, ArH), 11.02 (s, 1H, NH); ESI-MS m / z: 532.2 [M+H] + , 554.2 [M+Na] + ; IR (KBr, v ): 3445, 3179, 3067, 3002, 2932, 2837, 2560, 2300, 2035, 1961, 1896, 1728, 1652, 1616, 1537, 1507, 1487, 1434, 1363, 1337, 14, 129 1089, 1024, 953, 897 cm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com