Method for separating and recovering copper, nickel and iron from electroplating sludge
A technology for electroplating sludge, separation and recovery, which is applied in the field of solid waste resource utilization, can solve the problems of difficult sludge disposal, and achieve the effects of short process, simple operation and low basic investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
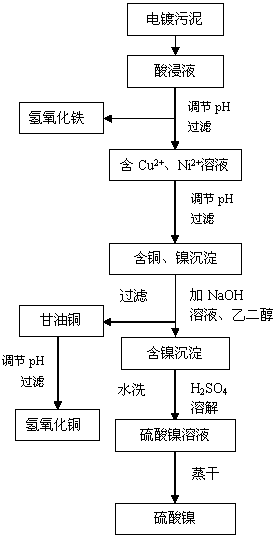
[0020] Add 200 mL of 1 mol / L sulfuric acid solution to 20 g of dried electroplating sludge, dissolve for 3 hours, and filter the leaching solution that contains Cu 2+ 、Ni 2+ The solution was adjusted to pH 3 with NaOH, allowed to settle for 8 hours, and 93% ferric hydroxide precipitate was obtained after filtration. Sodium hydroxide was used to adjust the pH of the filtrate to 7, and the precipitate was allowed to stand for 3 hours, and the filtered precipitate was washed three times with deionized water (liquid-solid ratio 20). The precipitate and deionized water are mixed with a liquid-solid ratio of 20, and the pH is adjusted to 9 with NaOH. After adding glycerol with 1.2 times the stoichiometric number of copper ions in the precipitate and stirring, a deep blue solution is formed, and after filtration, nickel hydroxide is obtained. Precipitate; The purple blue solution that obtains is the copper hydroxide solution that adds 1mol / L sulfuric acid to adjust pH 7 to regenerat...
Embodiment 2
[0022] Add 200 mL of 5 mol / L sulfuric acid solution to 20 g of dried electroplating sludge, dissolve for 3 hours, and filter the leaching solution that contains Cu 2+ 、Ni 2+ The solution was adjusted to pH 5 with NaOH, left to settle for 24 hours, and 95% ferric hydroxide precipitate was obtained after filtration. Sodium hydroxide was used to adjust the pH of the filtrate to 8, and the precipitate was allowed to stand for 5 hours, and the filtered precipitate was washed three times with deionized water (liquid-solid ratio 20). The precipitate and deionized water are mixed with a liquid-solid ratio of 20, and the pH is adjusted to 11 with NaOH. After adding glycerol with 1.6 times the stoichiometric number of copper ions in the precipitate and stirring, a deep blue solution is formed, and after filtration, nickel hydroxide is obtained. Precipitate; The purple blue solution that obtains is glycerol copper solution and adds 5mol / L sulfuric acid to adjust pH=8 and regenerates and...
Embodiment 3
[0024] Add 200mL of 3mol / L sulfuric acid solution to 20g of dried electroplating sludge, dissolve for 5h, and filter the leaching solution that contains Cu 2+ 、Ni 2+ The solution was adjusted to pH 4 with NaOH, left to settle for 15 hours, and 92% ferric hydroxide precipitate was obtained after filtration. Sodium hydroxide was used to adjust the pH of the filtrate to 7.5, and the precipitate was allowed to stand for 4 hours, and the filtered precipitate was washed 3 times with deionized water (liquid-solid ratio 20). The precipitate and deionized water are mixed with a liquid-solid ratio of 20, and the pH is adjusted to be 10 with NaOH. After adding glycerol with 1.4 times the stoichiometric number of copper ions in the precipitate and stirring, a deep blue solution is formed, and after filtration, nickel hydroxide is obtained. Precipitate; The purple blue solution that obtains is glycerol copper solution and adds 3mol / L sulfuric acid to adjust pH=7.5 and regenerates precipit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com