Method for preparing lactone through catalyzing oxidation of ketone compound, and its special catalyst
A compound and catalyst technology, applied in the field of catalyzing the oxidation of ketone compounds to prepare lactones, can solve the problem of large dosage of auxiliary agents, and achieve the effects of reducing dosage, improving utilization rate and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
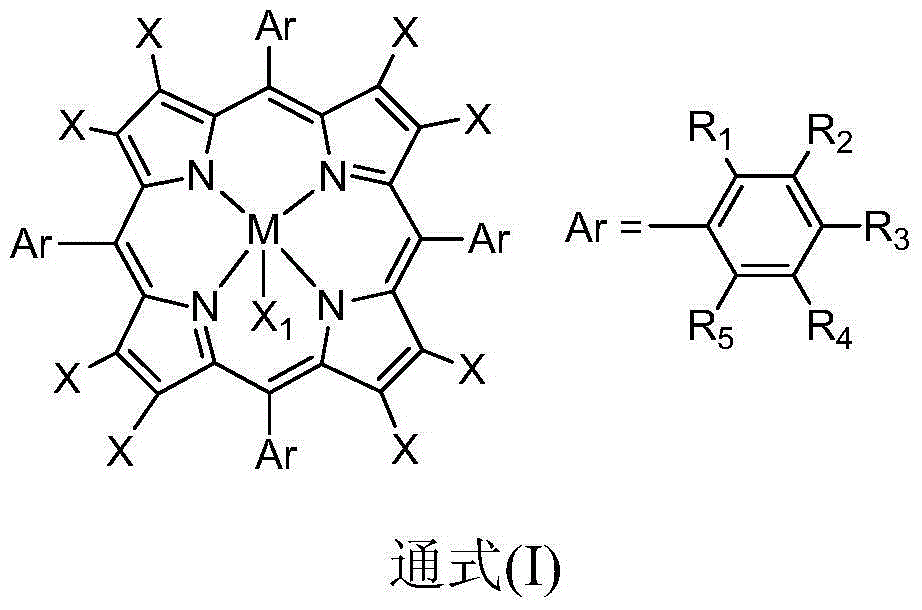
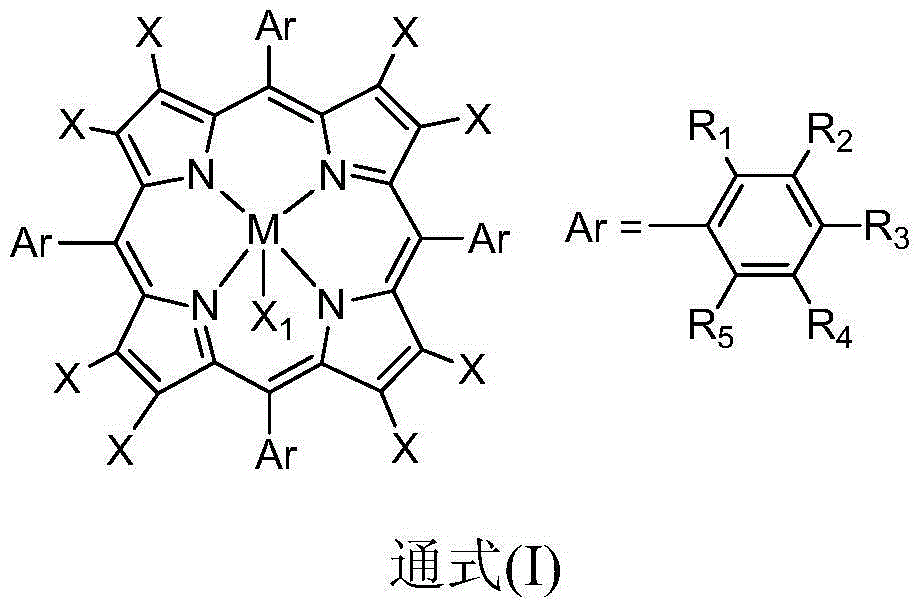
[0020] Take 0.020g of carboxylated multi-walled carbon nanotubes (c-MWCNTs), add 20mL of anhydrous N,N-dimethylformamide into a 50mL three-necked flask, and ultrasonicate the reaction solution for 3h. After the reactants are uniformly dispersed, the Add 0.100g FeTPPCl into the flask, heat it in an oil bath to reflux, cool the reaction solution to room temperature after 8 hours, filter it with a 0.22 μm polytetrafluoroethylene microporous membrane, wash it with tetrahydrofuran for more than three times until it is colorless, and then The black substance was dispersed into N,N-dimethylformamide again, ultrasonicated for 20 minutes, filtered through a 0.22 μm polytetrafluoroethylene microporous membrane, washed with tetrahydrofuran until the filtrate was colorless, and could be obtained after repeated operations Unreacted metalloporphyrins are removed. Disperse the obtained black substance into N,N-dimethylformamide again, ultrasonicate it, place it on a centrifuge and centrifuge...
Embodiment 2
[0022] In 10mL containing 5mg metalloporphyrin-carboxylated multi-walled carbon nanotubes (M=Fe, X=F, R 1 , R 2 , R 3 , R 4 , R 5 =H,X 1 In the 1,2-dichloroethane solution of F), add 2mmol of cyclohexanone and 2mmol of benzaldehyde, feed oxygen, and stir the reaction at a temperature of 50°C for 2 hours. After detection and analysis, the cyclohexanone The conversion rate is 89%, and the selectivity of ε-caprolactone is greater than 99%.
Embodiment 3
[0024] In 10mL containing 10mg metalloporphyrin-carboxylated single-walled carbon nanotubes (M = Fe, X = F, R 1 , R 2 , R 3 , R 4 , R 5 =H,X 1 In the 1,2-dichloroethane solution of F), add 2mmol of 4-methylcyclohexanone and 6mmol of benzaldehyde, and stir the reaction at a temperature of 60°C for 5 hours. After detection and analysis, 4-methyl The conversion rate of cyclohexanone is 92%, and the selectivity of 4-methylcyclocaprolactone is greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com