Intracellular delivery
A cell and cell membrane technology, applied in the field of intracellular delivery, can solve problems such as difficulty in developing methods and high specificity, achieve accurate and large-scale delivery, and reduce experimental noise
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
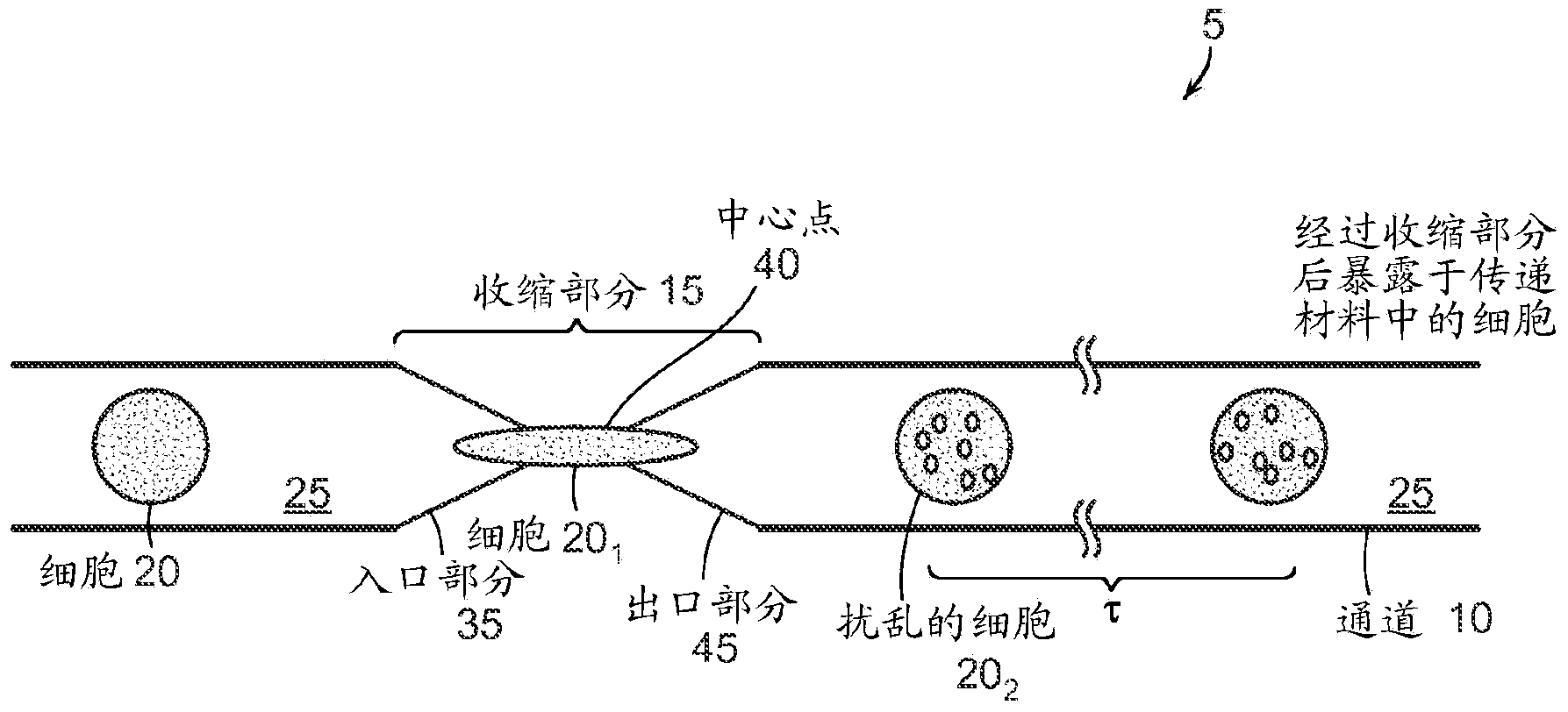
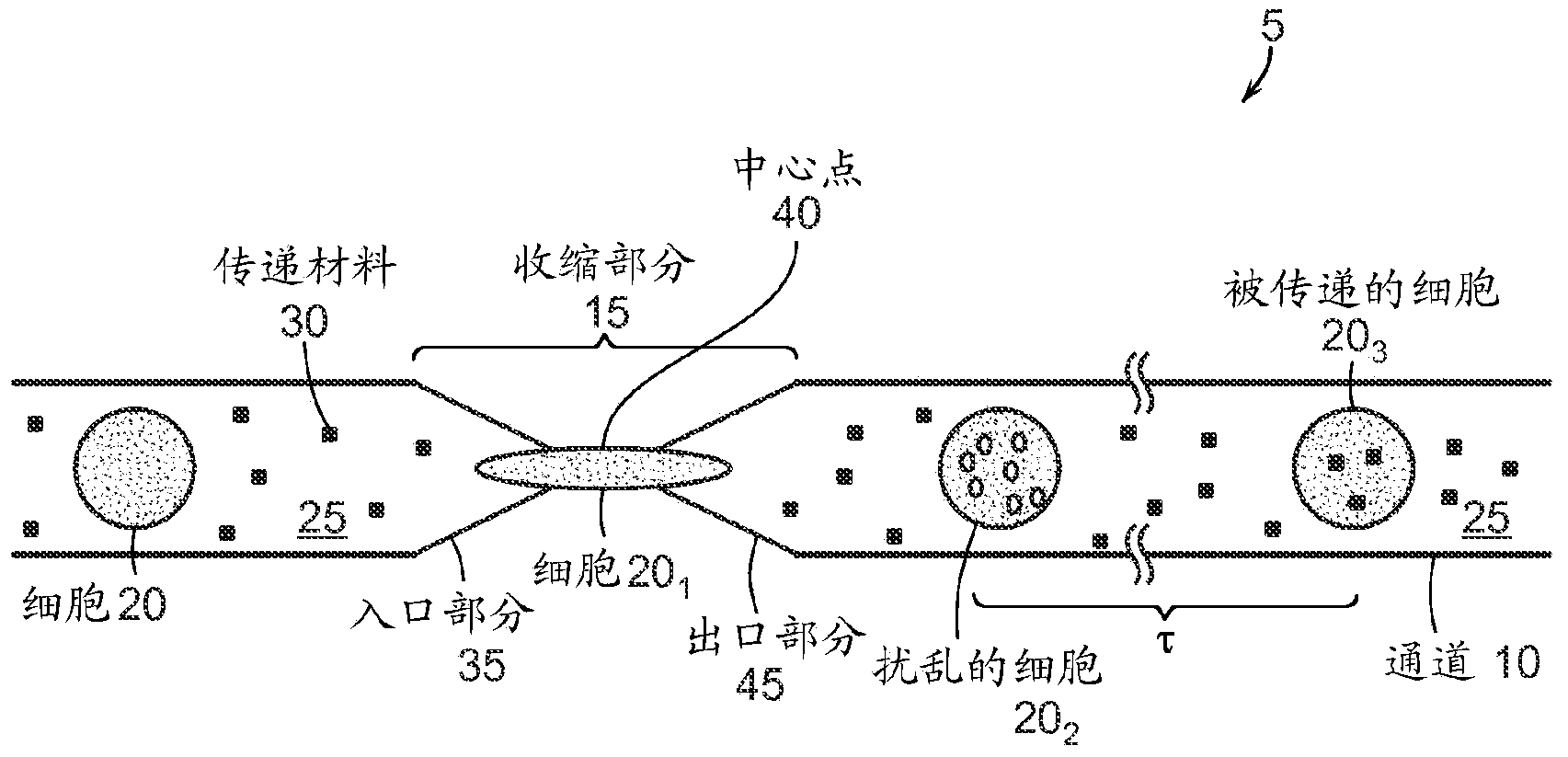
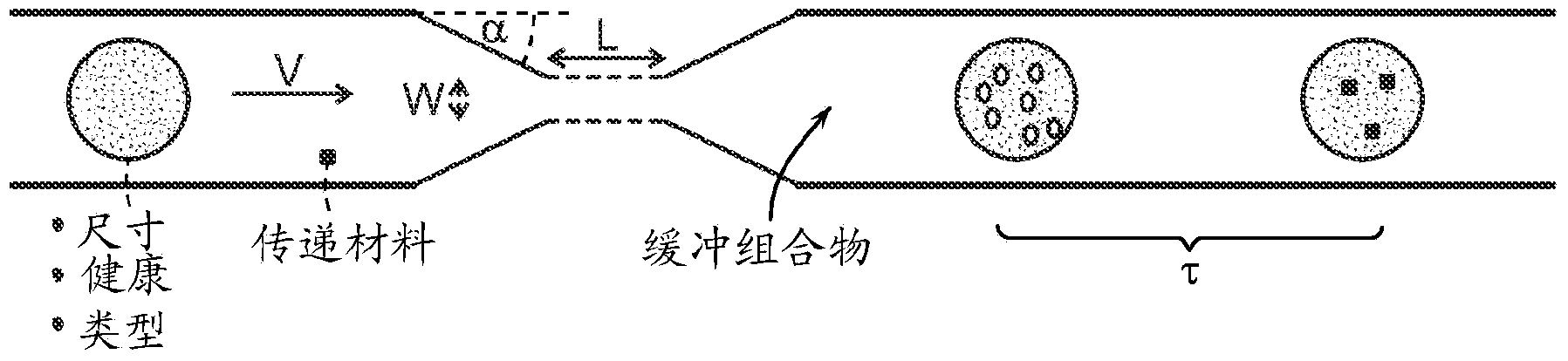
Image
Examples
Embodiment 1
[0137] Example 1 - Delivery of Functionally Engineered Nanoparticles
[0138] Engineered nanoparticles have great potential as live cell imaging tools, therapeutic molecule delivery agents or even as a method of manipulating living cells by using external means such as optical or magnetic fields (Howarth, M., et al. Monovalent, reduced-size quantum dots for imaging receptors on living cells (monovalent small-size quantum dots for imaging receptors in living cells). Nature Methods 5, 397-399 (2008)). However, many of these potential applications require delivery of nanomaterials into the cytoplasm of cells. Most nanoparticles, such as QDs, need to be passivated by polymers to enable the nanoparticles to dissolve in aqueous media, which often hinders the passive diffusion of nanoparticles across cell membranes.
[0139] Microinjection of nanoparticles was considered unfeasible due to lack of specialized instruments and low throughput while electroporation enables intracellula...
Embodiment 2
[0162] Example 2 - Delivery of macromolecules
[0163] Intracellular delivery of macromolecules is a critical step for both therapeutic and research applications. Nanoparticle-mediated DNA and RNA delivery can be useful, for example, in gene therapy, while protein delivery can be used to affect cellular function both in the clinic and in the laboratory. Other materials, such as small molecules, quantum dots, or gold nanoparticles, can be delivered into the fluid of cells for applications including cancer therapy, intracellular labeling, and single-cell tracking.
[0164] To illustrate the versatility of the technique, model dextran molecules were delivered into several cell types: DC2.4 dendritic cells, neonatal human foreskin fibroblasts (NuFF) and mouse embryonic stem cells (mESC), the delivery efficiencies obtained Respectively as high as 55%, 65% and 30%. Initial implementations also showed successful delivery in primary lymphocytes, macrophages, and dendritic cells fr...
Embodiment 3
[0201] Example 3 - Stem Cells and Immune Cells
[0202] Proteins, nanoparticles, siRNA, DNA and carbon nanotubes were successfully delivered to 11 different cell types, including embryonic stem cells and immune cells. Indeed, the devices and methods of the present invention are able to deliver structurally diverse materials and their availability into primary cells that are difficult to transfect, suggesting broad research and clinical applications of the present methods.
[0203] In Example 3, each device consisted of 45 identical microfluidic channels arranged in parallel, including one or more constrictions, etched on a silicon chip and sealed with a layer of borosilicate glass. The width and length of each constriction (described in more detail below) are 4-8 microns and 10-40 microns, respectively. The devices of Example 3 were typically operated at a throughput rate of 20,000 cells / second, each device was capable of producing close to one million processed cells befor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com