Pharmaceutical composition treating tumors
A composition and drug technology, applied in the fields of biology and medicine, can solve problems such as tumors that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Preparation of monoclonal antibody master solution: Weigh 10 mg of chLym-1 monoclonal antibody freeze-dried powder and dissolve it in 1 ml of 0.02M PBS solution with pH=7.4. Stir well and filter through a 0.1 μm sterile filter. Divide into 10 tubes for storage. Diluted to 10μg / ml for in vitro tests.
[0040] Preparation of rituximab stock solution: Weigh 10mg of rituximab freeze-dried powder and dissolve it in 1ml of 0.02M PBS solution with pH=7.4. Stir well and filter through a 0.1 μm sterile filter. Divide into 10 tubes for storage. Diluted to 10μg / ml for in vitro tests.
[0041] Preparation of trastuzumab stock solution: Weigh 10mg of lyophilized powder of trastuzumab and dissolve in 1ml of 0.02M PBS solution with pH=7.4. Stir well and filter through a 0.1 μm sterile filter. Divide into 10 tubes for storage. Diluted to 10μg / ml for in vitro tests.
[0042] Autophagy inhibitor drug configuration
[0043] (1) Preparation of chloroquine solution: Dissolv...
Embodiment 1
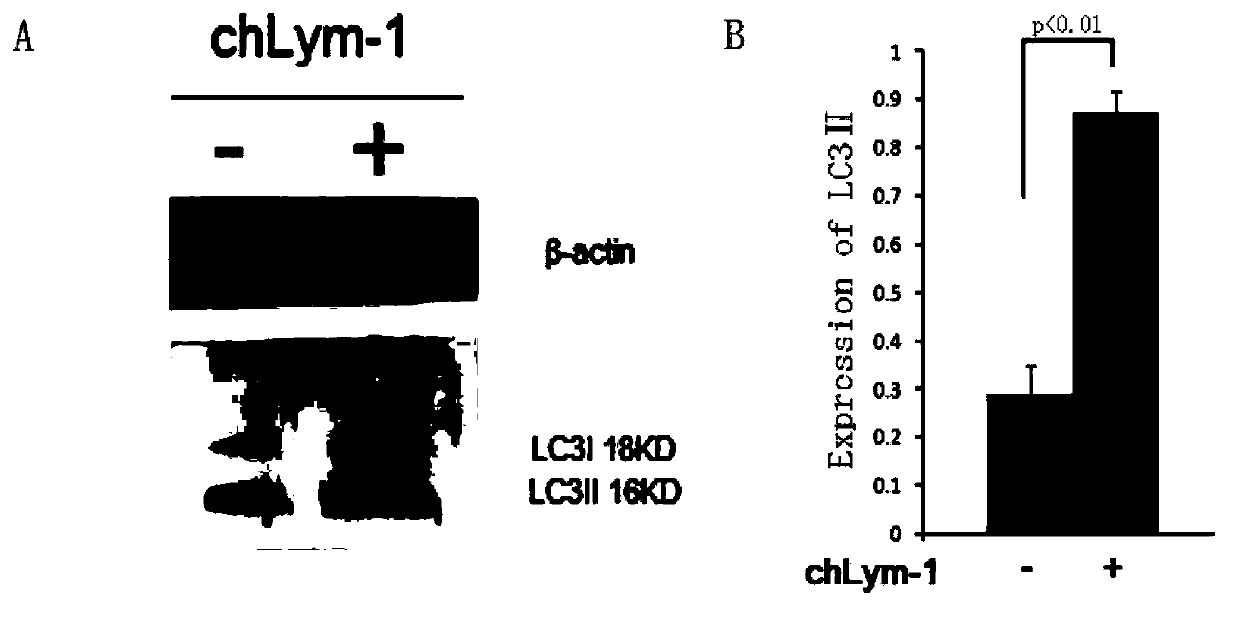
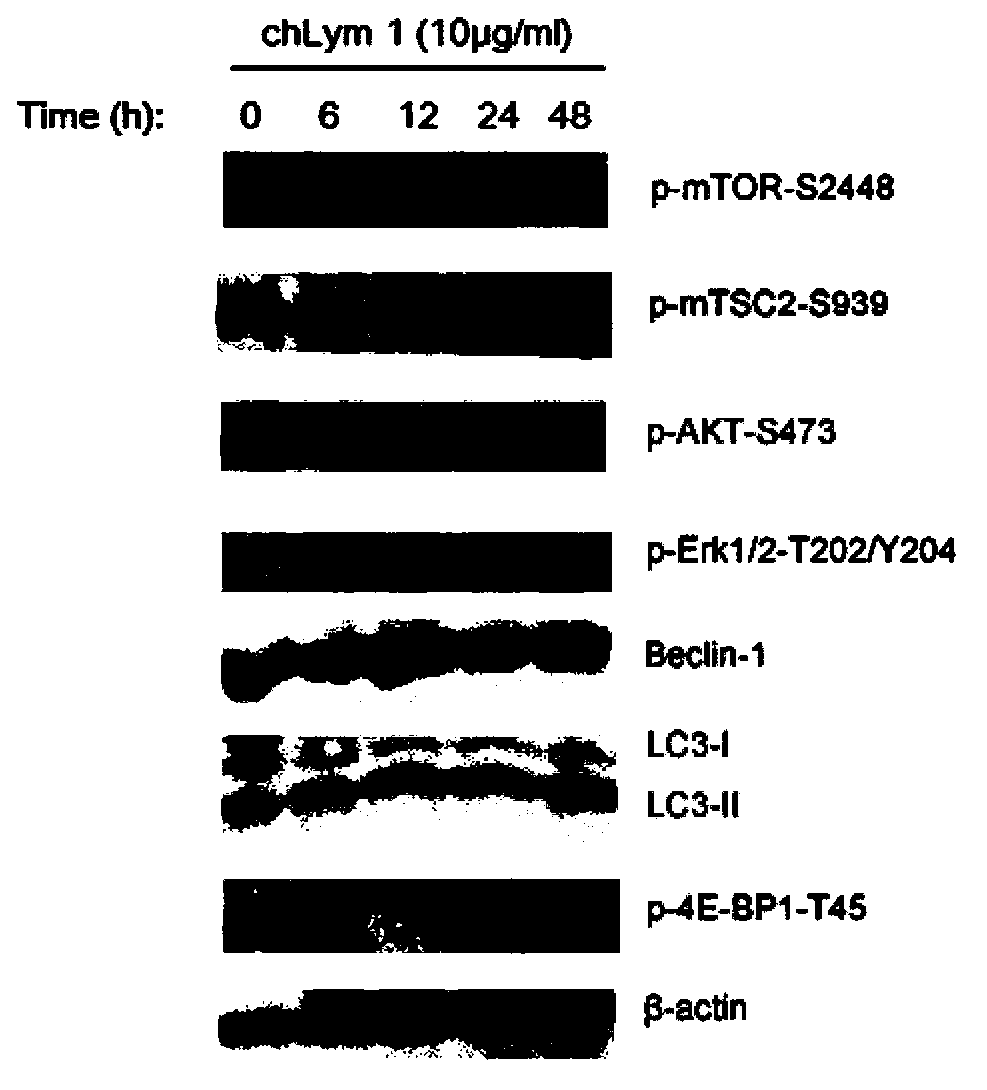
[0052] Example 1 chLym-1 monoclonal antibody induces up-regulation of LC3-II expression in Raji cells
[0053] The Raji cells treated with 10 μg / ml chLym-1 monoclonal antibody for 24 hours and the control group Raji were lysed with Western Blot and IP lysate to extract the total protein of the cells for protein quantification, and 50 μg of protein per well was loaded for protein electrophoresis and analyzed. Transfer membrane for Western blot, and use ECL chemiluminescence kit for chemiluminescence, the results are as follows figure 1 As shown: the expression of autophagy-related protein LC3Ⅱ in Raji cells treated with 10 μg / ml chLym-1 monoclonal antibody for 24 hours was higher than that of the control group, and the grayscale processing software IQuentTL analysis found that there was a very significant difference between the two ( p <0.01), due to the positive correlation between the expression of LC3Ⅱ and the number of autophagosomes, the higher the expression of LC3Ⅱ, the ...
Embodiment 2
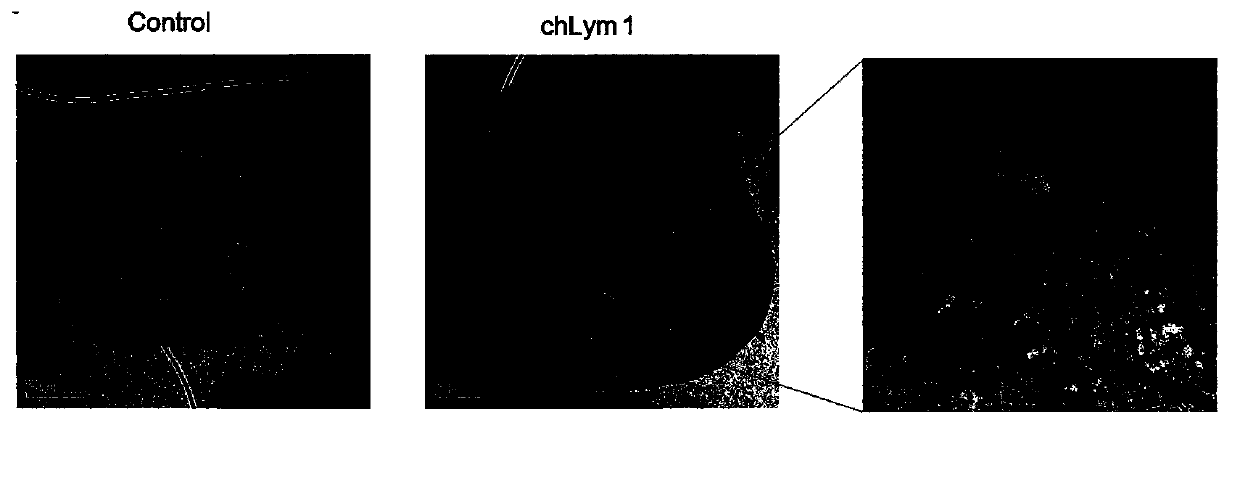
[0056] Example 2 chLym-1 monoclonal antibody induces autophagosome aggregation in Raji cells
[0057] Raji cells intervened by chLym-1 monoclonal antibody for 24 hours were collected, fixed, embedded, sectioned, and stained, and then their ultrastructure was observed under an electron microscope. 10μg / ml chLym-1 monoclonal antibody intervened for 24 hours, and the nucleus of Raji cells was severely deformed. Under the magnification of 8000×, many vacuole-like structures were found in the extranuclear area, and under the magnification of 50000×, a large number of Obvious bilayer membrane structure (such as image 3 shown), according to literature reports, the diameter of autophagosomes is generally between more than 300 nanometers and several microns, which is consistent with the size of the double-membrane structure vacuoles shown in the figure of this example. The analysis confirms that the arrow points to The double-membrane-like vacuoles were autophagosomes. Compared wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com