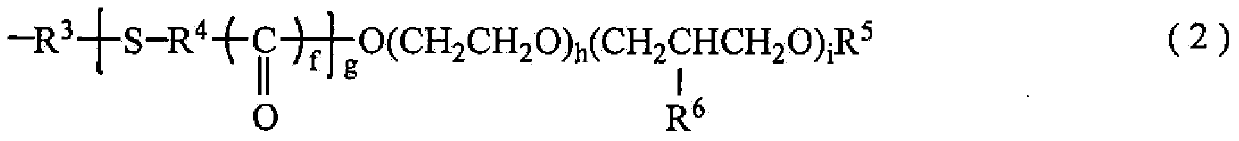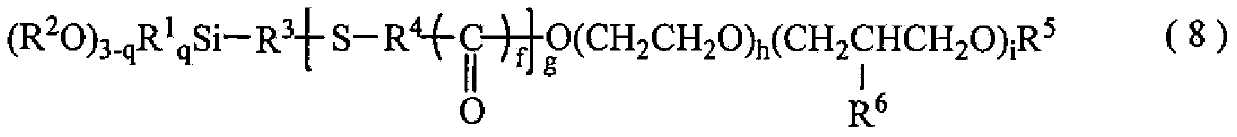Polyether modified alkoxy silane containing organo-functional group and manufacute method for the same
An organosiloxane, alkoxysilane technology, applied in textiles and papermaking, coatings, adhesives, etc., can solve the problems of reduced reactivity, no co-modification, and intolerant of use, and achieves inhibition of coloration and residue. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] [Example 1] (Synthesis of polyether-modified methoxysiloxane containing mercapto group-1)
[0163] 25.0 g of 3-mercaptopropyl trimethoxysilane (12.7 × 10 -2 mol), 28.8g (4.25×10 -2 mol) and diacetone alcohol 17.0g, 2.67g of 0.05N hydrochloric acid aqueous solution (water: 14.8×10 -2 mol, hydrochloric acid: 1.34×10 -4 mol), and then matured for 2 hours.
[0164] (CH 3 O) 3 Si-C 3 h 6 -O(CH 2 CH 2 O) 11 CH 3 (19)
[0165] Next, add 1.91 g of a 1% methanol solution of sodium acetate (sodium acetate: 2.33×10 -4 mol), the temperature was raised to 70°C for 2 hours of aging.
[0166] Next, heating under reduced pressure at 100° C. and 10 mmHg for 2 hours, distilled off residual alcohol components and low boiling point components, and filtered to obtain a liquid organosiloxane 1 with a non-volatile content of 99.0% (yield: 42.7g, yield: 91%).
[0167] In this organopolysiloxane 1, in the above-mentioned average composition formula (1), Y is a 3-mercaptoprop...
Embodiment 2
[0170] [Example 2] (Synthesis of polyether-modified methoxysiloxane containing mercapto group-2)
[0171] 12.5 g of 3-mercaptopropyl trimethoxysilane (6.36 × 10 -2 mol) and 35.4g (6.36×10 -2 mol), 0.5 g of PERBUTYL-O [manufactured by NOF Corporation: tert-butyl peroxy-2-ethylhexanoate] was added with stirring, and aging was performed at 65° C. for 2 hours.
[0172] CH 2 =CH-CH 2 -O(CH 2 CH 2 O) 11 CH 3 (twenty three)
[0173] The resulting liquid was subjected to GPC (gel permeation chromatography) measurement in THF solvent. As a result, product peaks were confirmed within the retention time range of 28 minutes to 34 minutes. In addition, the peak derived from 3-mercaptopropyltrimethoxysilane almost disappeared in the retention time range of 36 minutes to 38 minutes. In addition, for the liquid generated above, the 1 H-NMR determination. As a result, the peak derived from the allyl group of the allylated polyether represented by the above formula (23) existin...
Embodiment 3
[0180] [Example 3] (Synthesis of polyether-modified methoxysiloxane containing mercapto group-3)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com