Directional synthetic method of 2, 5-di tertiary butyl hydroquinone
A di-tert-butyl hydroquinone, directional synthesis technology, applied in the field of fine chemicals and chemical synthesis, can solve the problems of low purity, high production cost, waste of raw materials, etc., achieve high selectivity and atom economy, reaction The effect of low equipment requirements and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
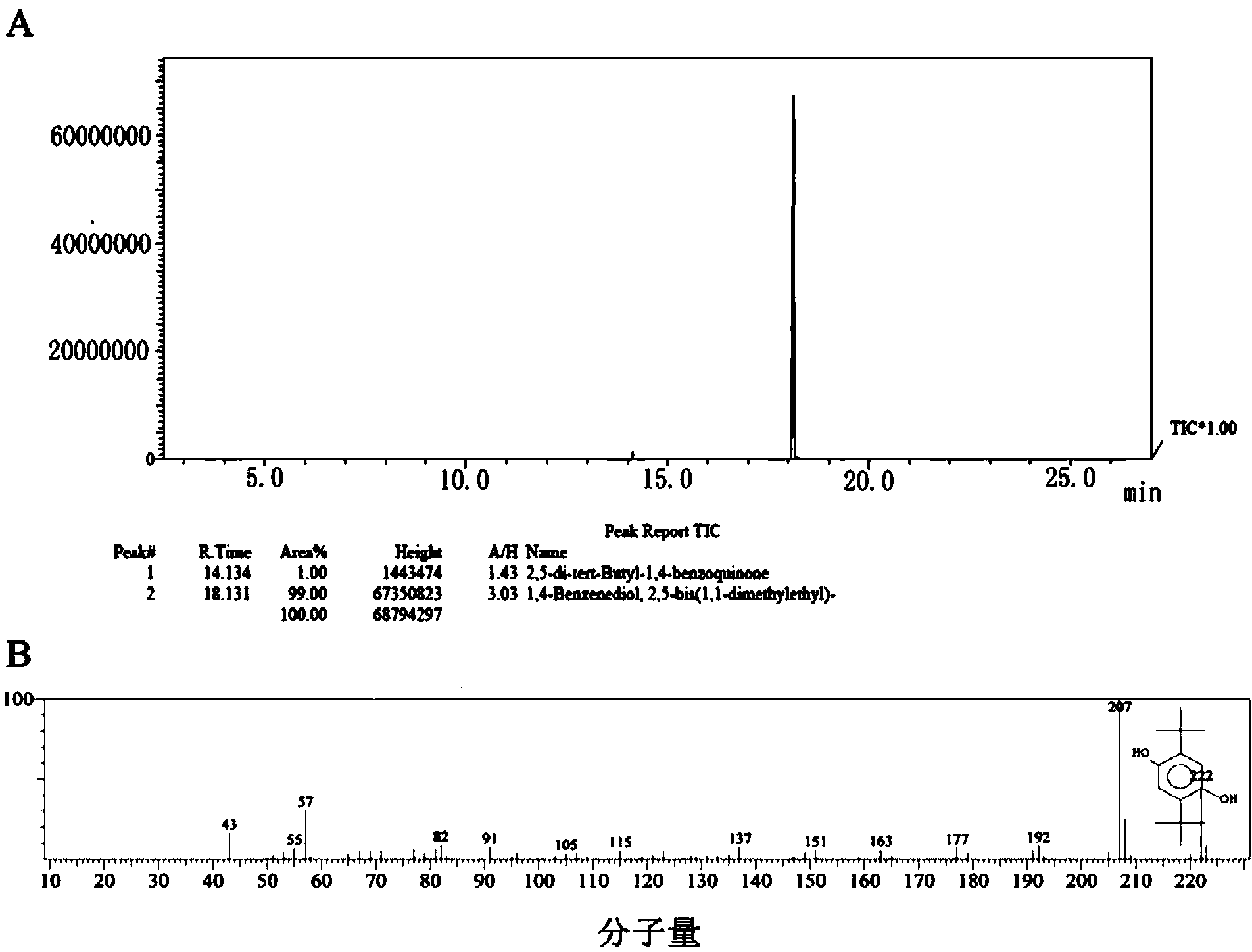
[0037] Mix 2.757g of hydroquinone with 0.142g of oxalic acid, add into a three-neck flask equipped with a thermometer, a stirrer, a condenser, and a constant pressure dropping funnel, add 2ml of water into the three-necked flask, stir, and heat to 82°C; Add 3.100 g of butanol from a constant pressure dropping funnel until the solid in the three-necked flask is completely dissolved, mix 8.030 g of tert-butanol with 2.457 g of concentrated sulfuric acid, slowly add it to the three-necked flask, and keep it at 82°C for 2 hours; stop the reaction and wait for the system to After cooling, filter, and wash the solid filter residue with 5 mL of water, repeat the washing 4 times, and dry to obtain 4.694 g of crude product 2,5-di-tert-butylhydroquinone, yield: 84.32%.
[0038] 3.898 g of the product 2,5-di-tert-butylhydroquinone was obtained by recrystallization, with a purity of over 99% and a comprehensive yield of 70.03%.
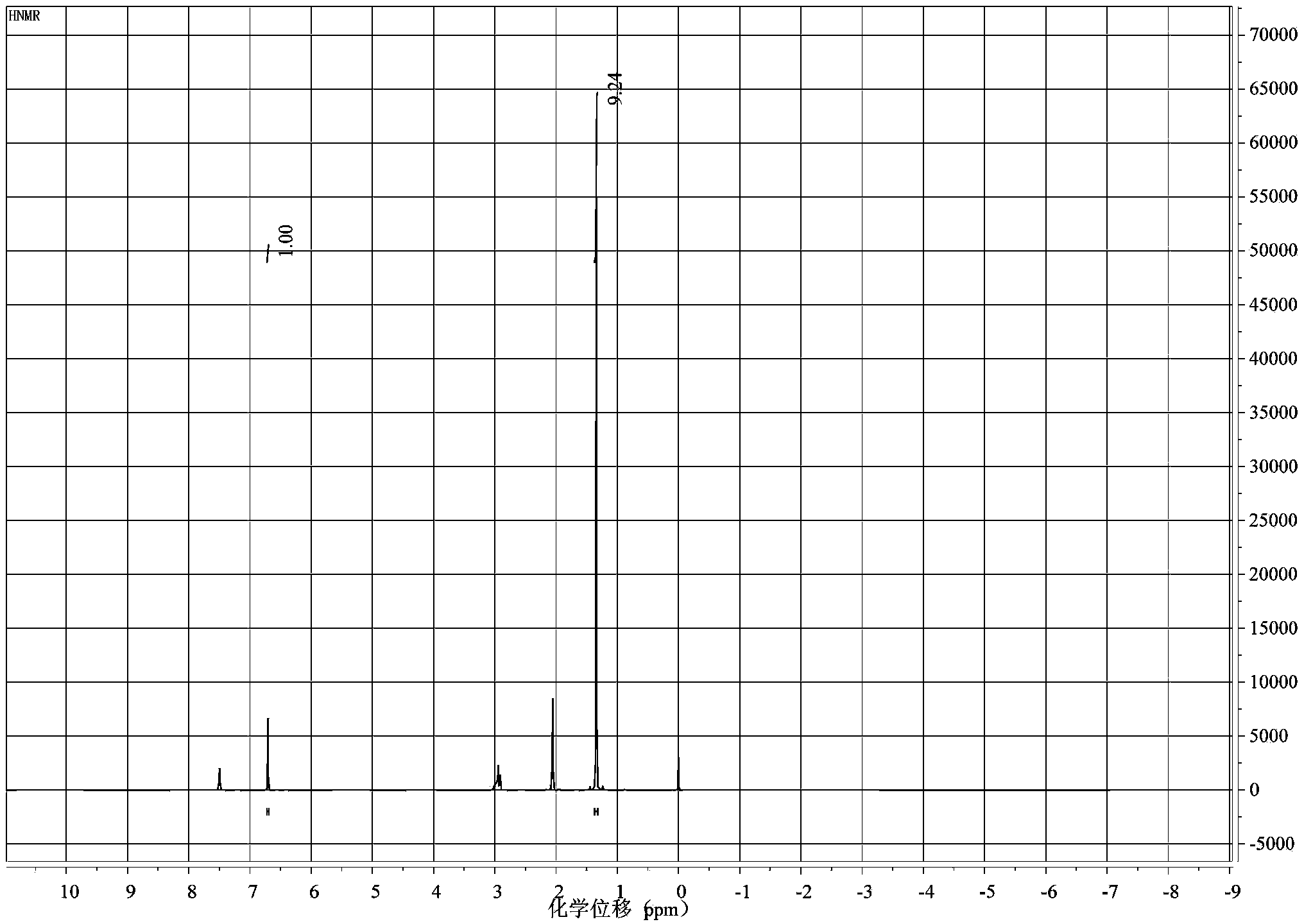
[0039] The product after recrystallization was taken, and a...
Embodiment 2
[0041]Take 2.732g of hydroquinone, put it into a three-necked flask equipped with a thermometer, a stirrer, a condenser, and a constant pressure dropping funnel, add 2ml of water into the three-necked flask, stir, and heat to 83°C; Add in a constant pressure dropping funnel until the solids in the three-necked flask are completely dissolved. Mix 8.152g of tert-butanol with 1.966g of concentrated sulfuric acid and 0.491g of phosphoric acid to make a mixed acid solution, slowly add it into the three-necked flask, and keep it at 83°C for 2 hours; stop the reaction , after the system was cooled, filtered, and the solid filter residue was washed with 5 mL of water, repeated washing 4 times, and dried to obtain 4.637 g of crude product 2,5-di-tert-butylhydroquinone, yield: 84.06%.
[0042] Recrystallization obtained 3.887 g of 2,5-di-tert-butylhydroquinone with a purity of over 99% and a comprehensive yield of 70.47%.
[0043] Get the product after recrystallization, carry out with ...
Embodiment 3
[0045] Mix 5.505g of hydroquinone with 0.228g of oxalic acid, add to a three-neck flask equipped with a thermometer, agitator, condenser, and constant pressure dropping funnel, add 4ml of water, stir, and heat to 86°C; 6.044g of tert-butanol Add from the dropping funnel until the solid in the three-necked flask is completely dissolved, mix 10.704g of tert-butanol with 4.971g of concentrated sulfuric acid, slowly add to the three-necked flask, and keep at 86°C for 2h; stop the reaction, and filter the solid after the system is cooled. The filter residue was washed with 10 mL of water, repeated washing 4 times, and dried to obtain 9.277 g of crude product 2,5-di-tert-butylhydroquinone, yield: 83.46%.
[0046] 7.894 g of the product 2,5-di-tert-butylhydroquinone was obtained by recrystallization, with a comprehensive yield of 71.32% and a purity of over 99%.
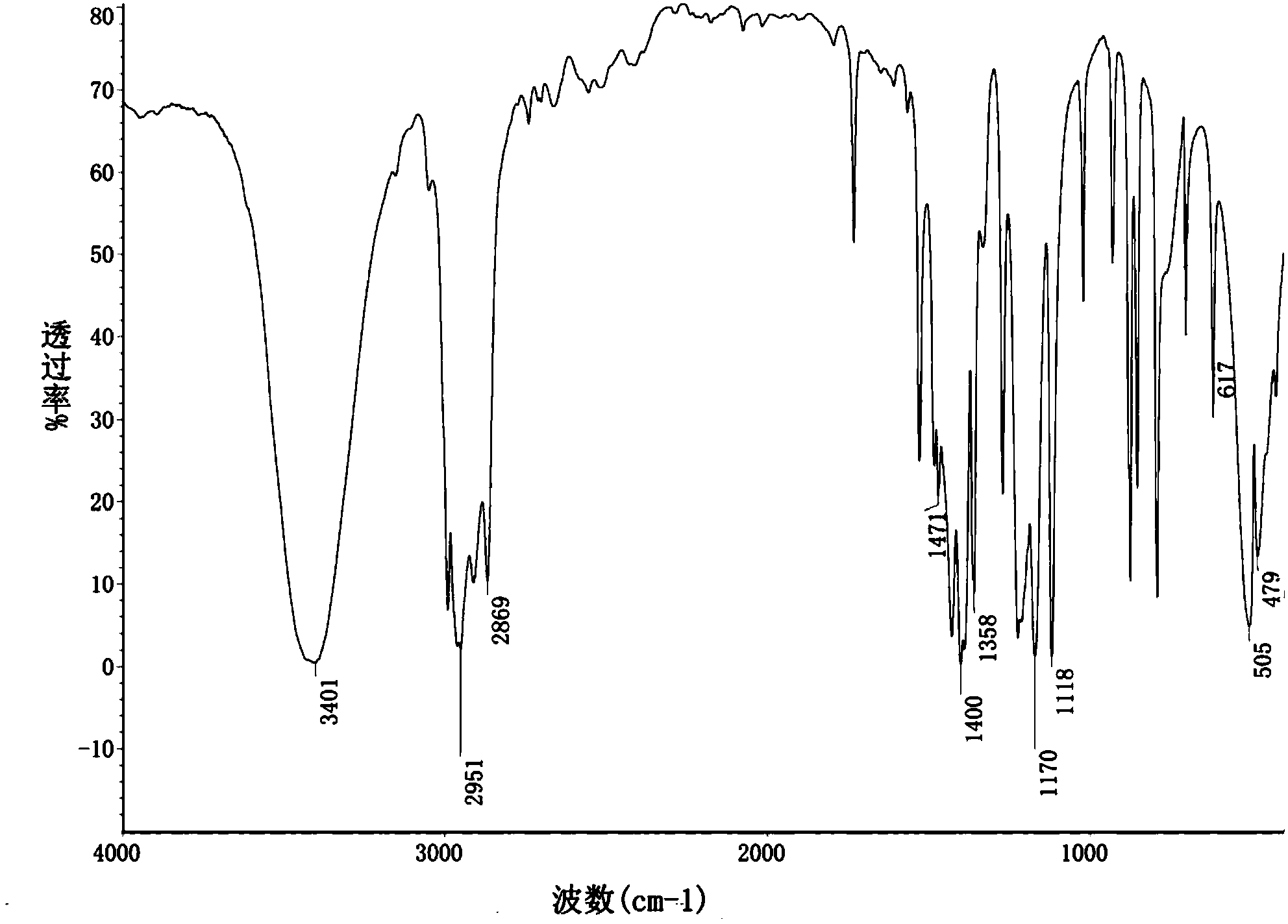
[0047] The product after recrystallization was taken, and it was detected by FT-IR by KBr tablet method. The detection in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com