Method for preparing phenylthioacetate
A phenylacetic acid and thiol technology, applied in sulfide preparation, organic chemistry and other directions, can solve the problems of difficulty in improving the purity of disposable products, many changes in impurities in the finished product, and high cost of secondary refining, so as to reduce the risk of reaction and reduce production. cost, the effect of reducing the pollution of the three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
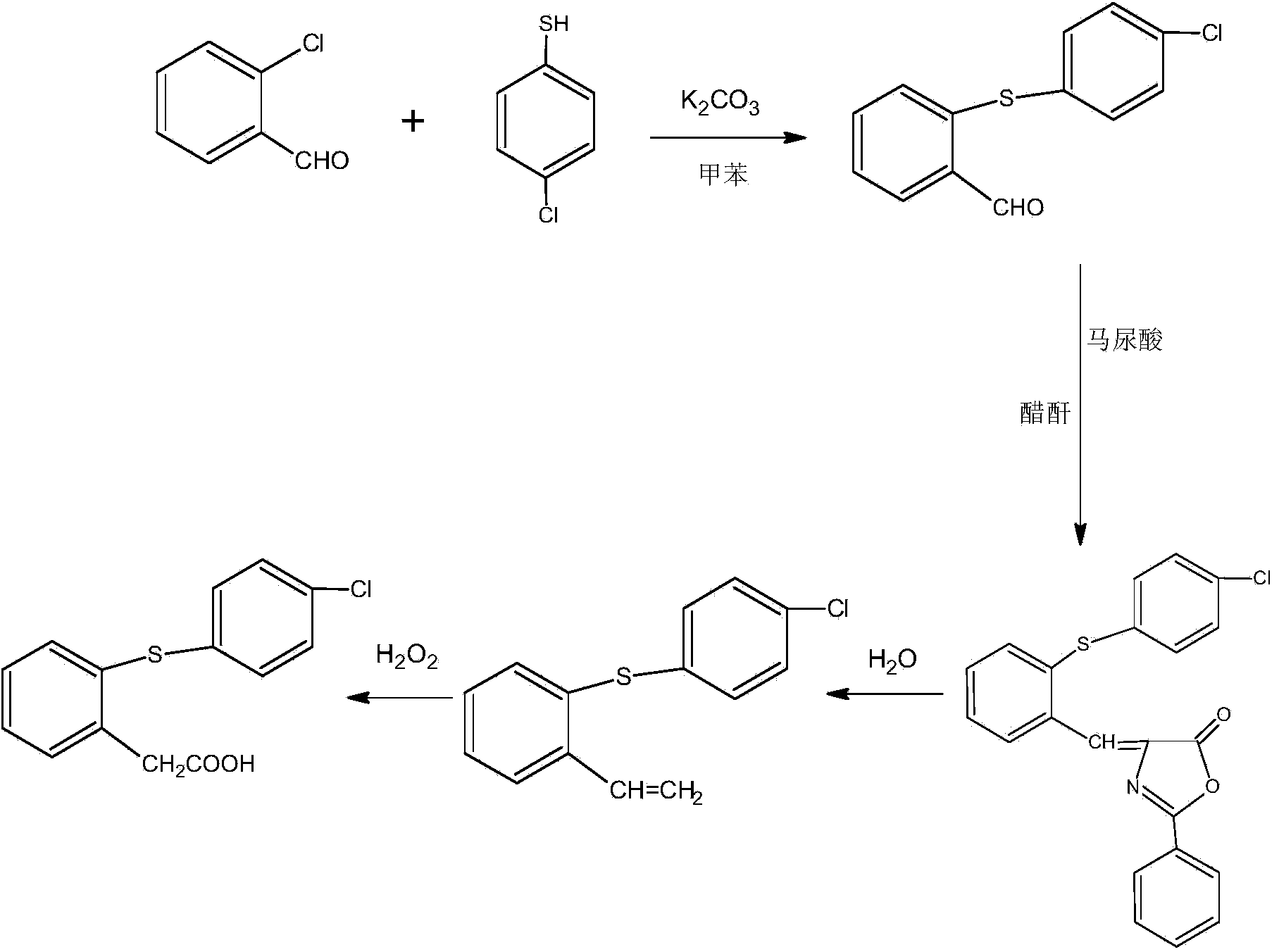
[0028] The present invention uses o-chlorobenzaldehyde and p-chlorothiophenol as raw materials to carry out the condensation reaction with o-chlorobenzaldehyde and p-chlorothiophenol as raw materials under the condition that toluene is used as a solvent, and then reacts with hippuric acid in acetic anhydride , a cyclization reaction occurs in the presence of sodium acetate, and finally hydrolyzed and oxidized to finally obtain the product thiophenylacetic acid; the specific steps are as follows:
[0029] Step 1, condensation reaction: In a 1000ml four-neck flask, put 500ml of toluene, 200g of o-chlorobenzaldehyde, 190g of p-chlorothiophenol, 10g of anhydrous potassium carbonate, heat up to 50-60°C, react for 3-4 hours, cool to below 5°C, filtered, washed with water to obtain thiobenzaldehyde, and dried to obtain 349 g of dry thiobenzaldehyde, with a yield of 98.5%.
[0030] Step 2, ring closure reaction: In a 2000ml four-necked flask, put 1000g of acetic anhydride, 349g of thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com