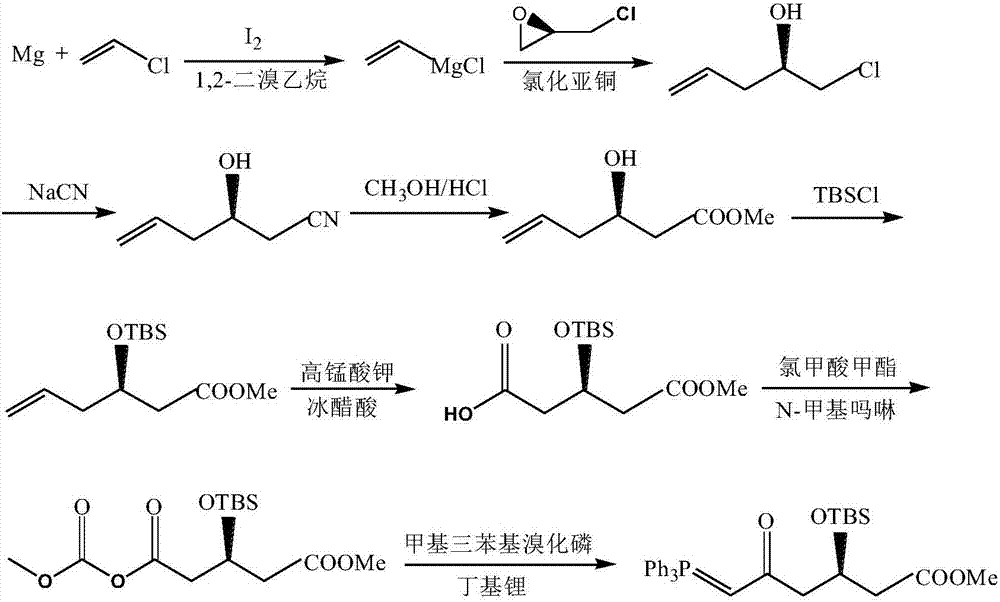
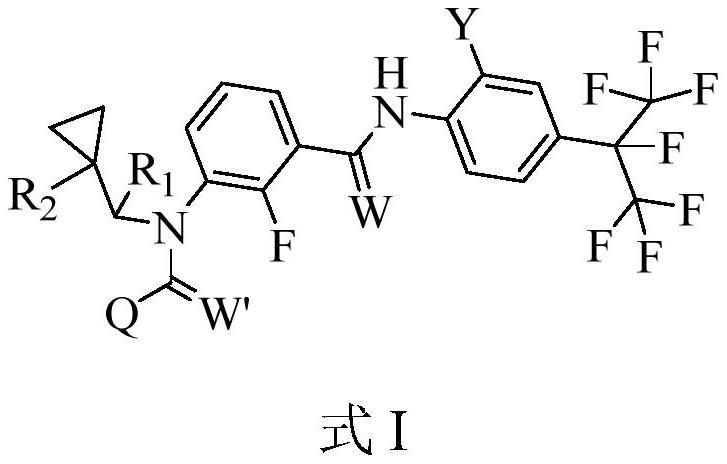
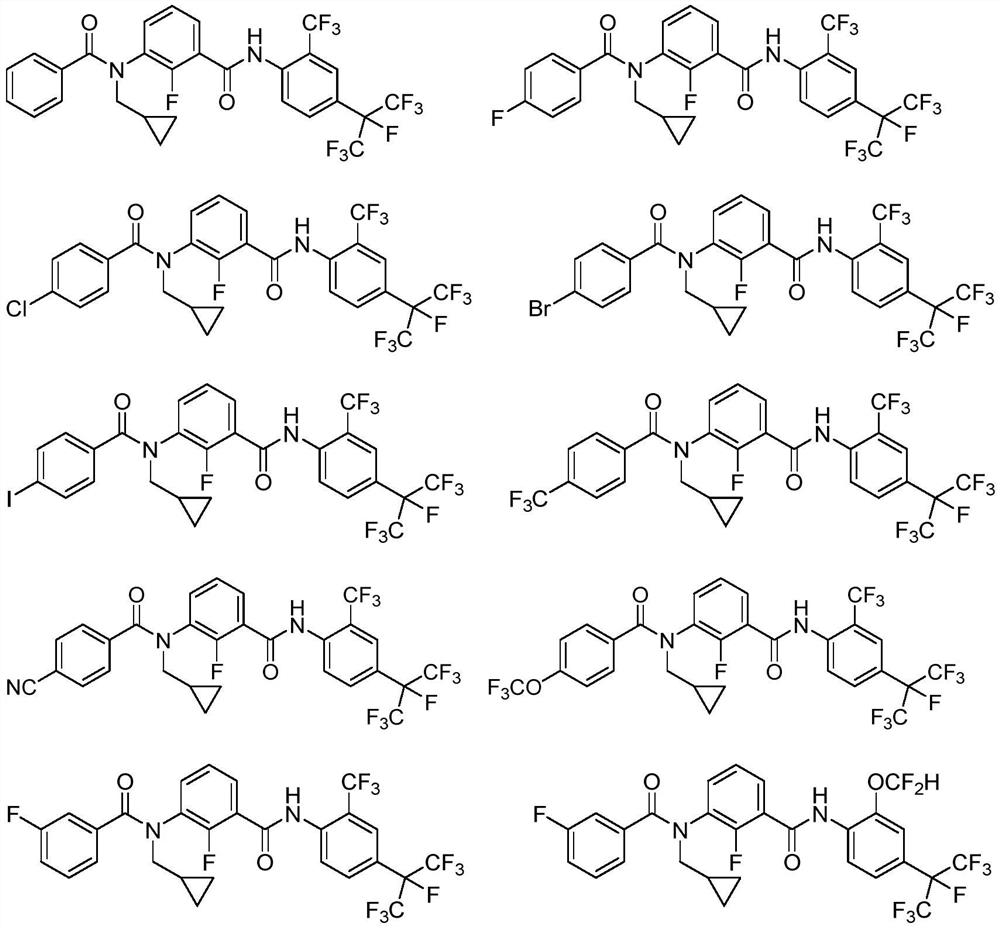
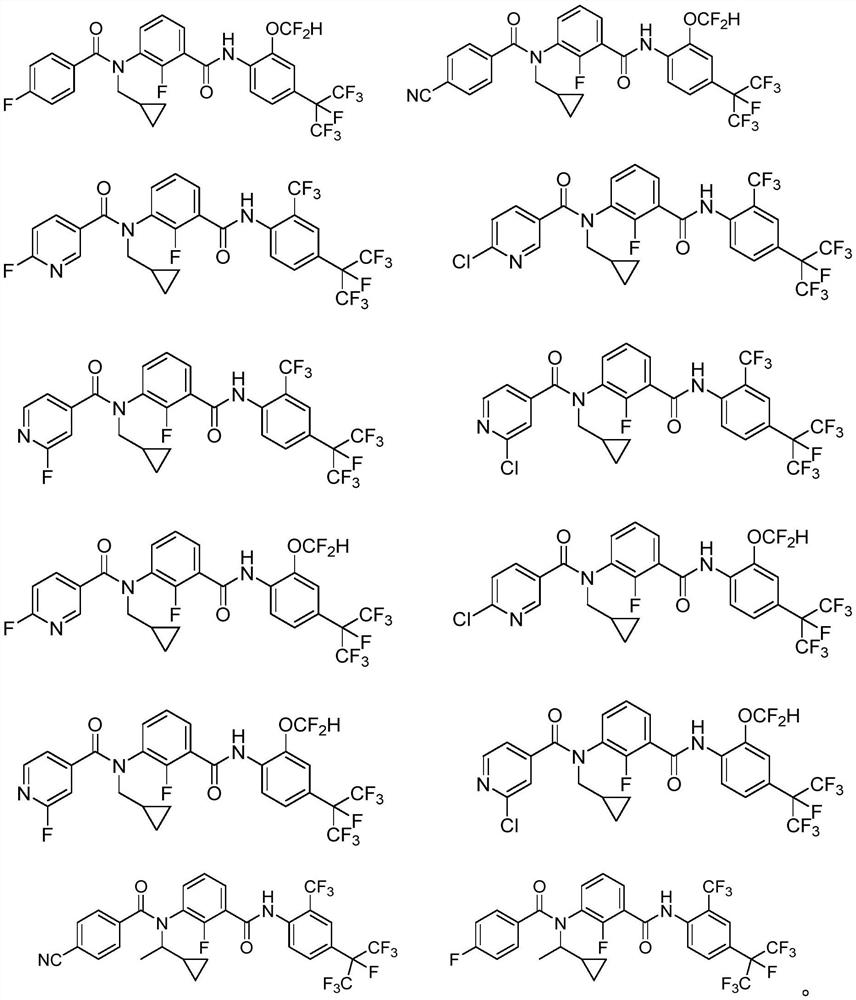
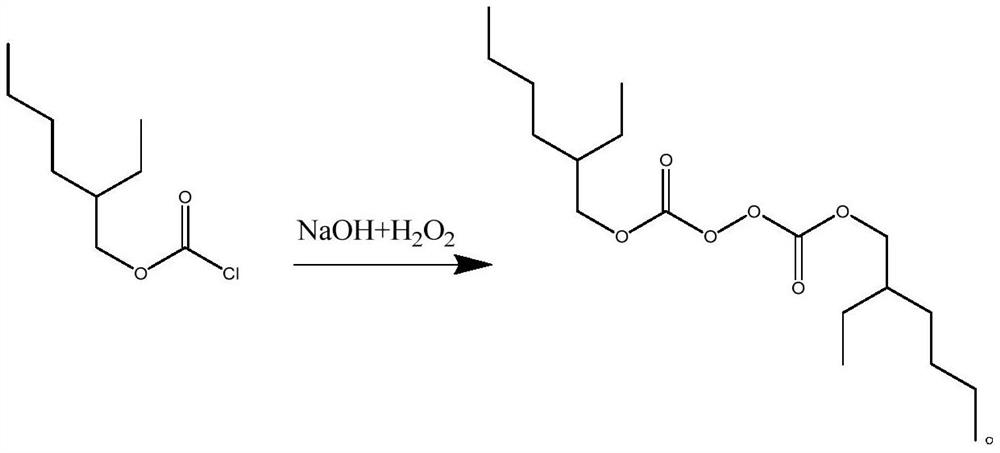
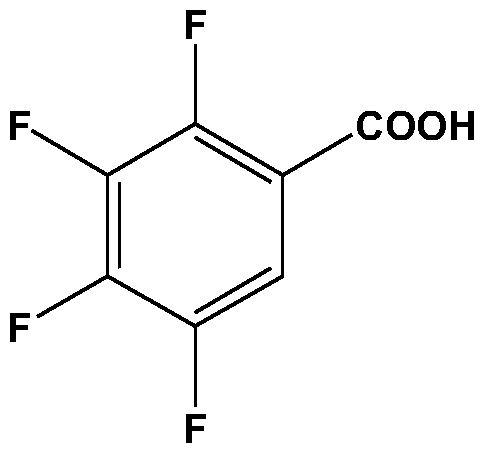
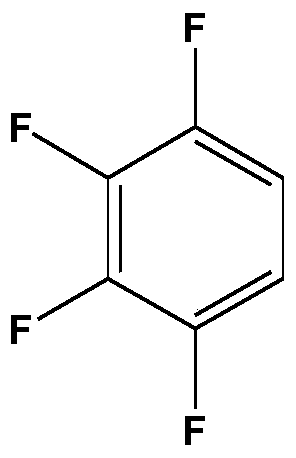
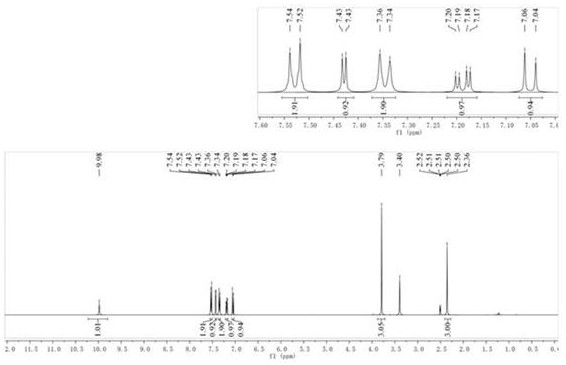
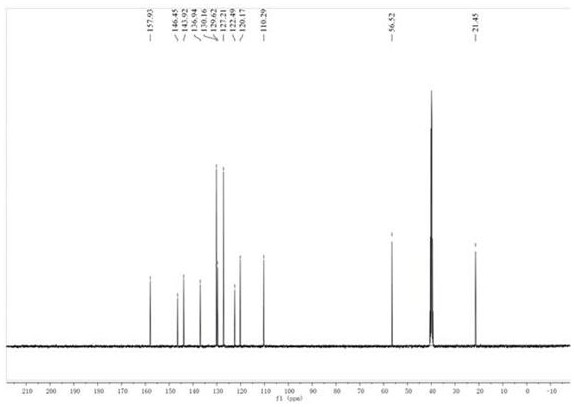
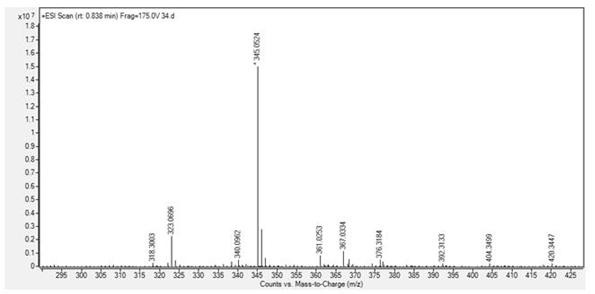
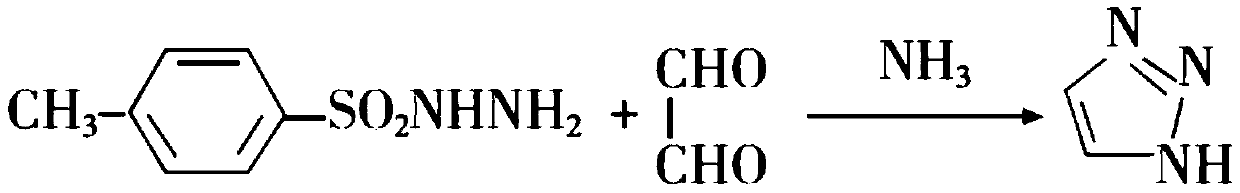Patents
Literature
31results about How to "Reduced risk of reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
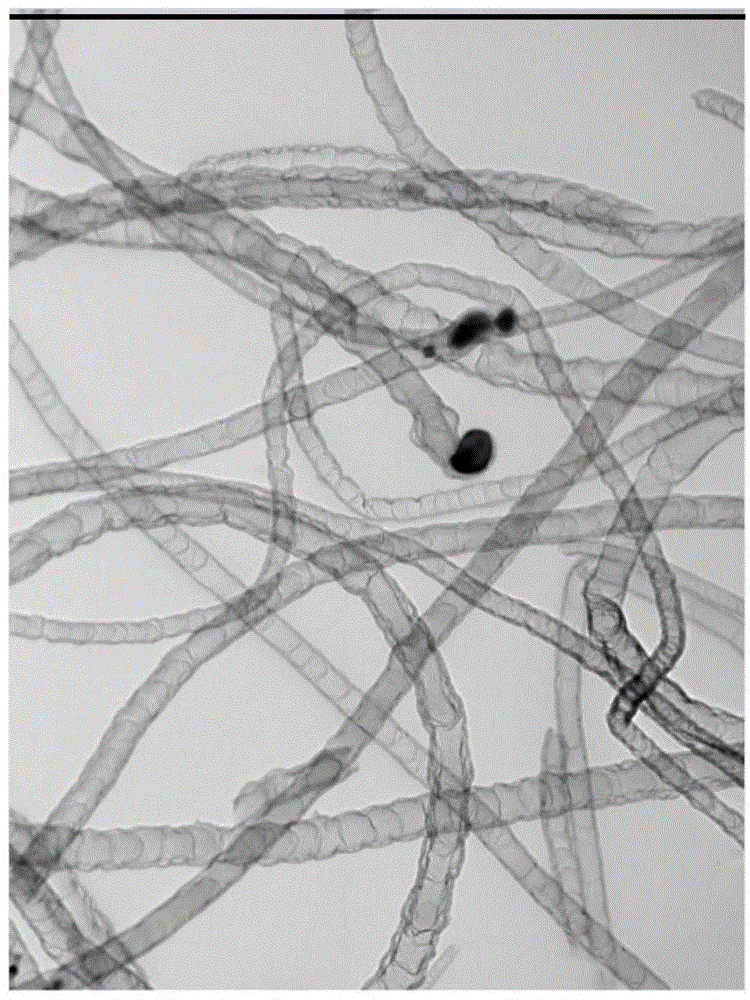
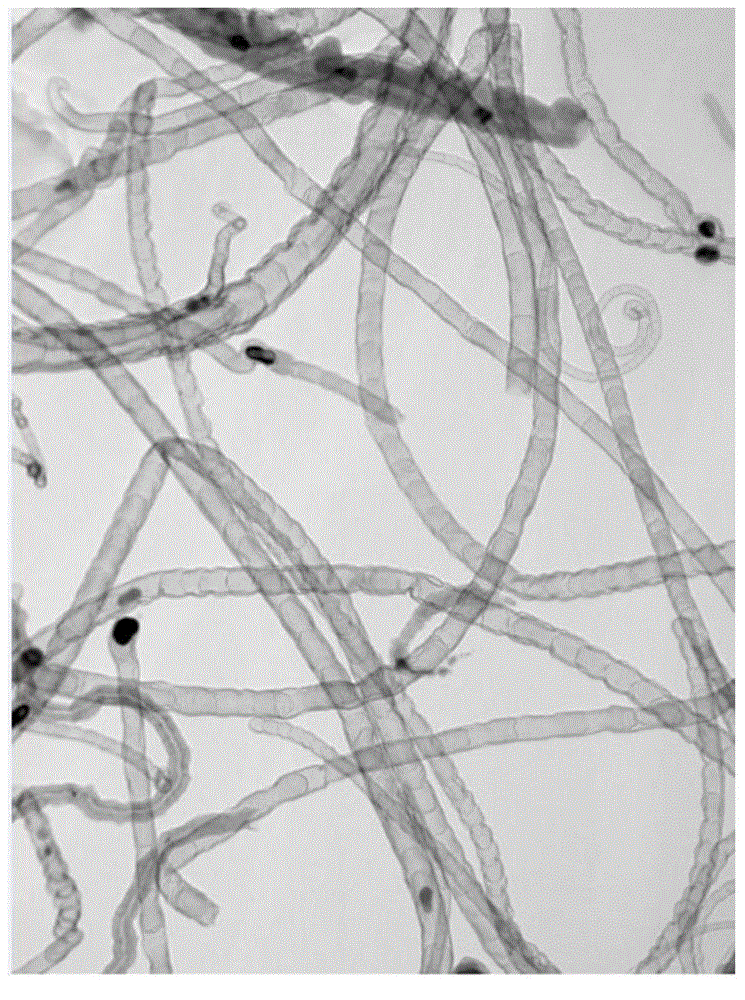
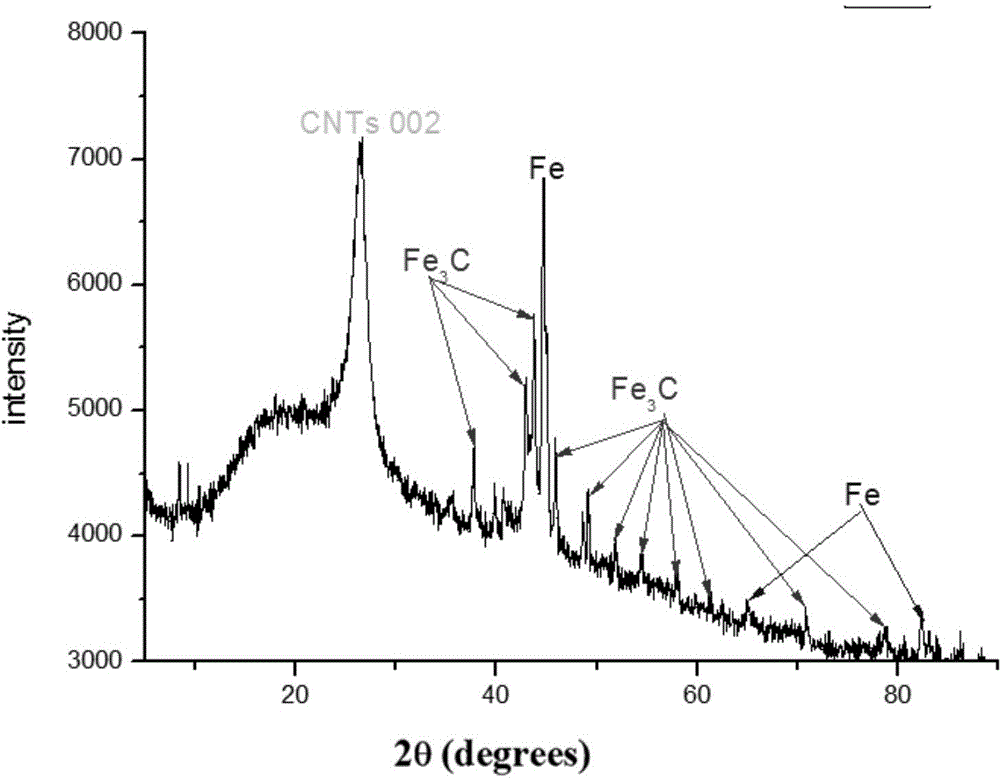
Method for preparation of bamboo-shaped carbon nanotube by ultrasonic atomization
InactiveCN105439118AControllable and adjustable processing capacityReduced risk of reactionMaterial nanotechnologyTube furnaceCarbon nanotube
The invention relates to a method for preparation of a bamboo-shaped carbon nanotube by ultrasonic atomization. The method includes the steps of: dissolving a metal salt and an organic ligand in certain solvent, conducting ultrasonic atomization to produce fine droplets, introducing an inert gas and loading the fine droplets into a tube furnace pre-heated to certain temperature, and carrying out reaction for a period of time to obtain the bamboo-shaped carbon nanotube. Compared with the existing technology for preparation of bamboo-shaped carbon nanotubes, the method provided by the invention can acquire the bamboo-shaped structure with good appearance, has the advantages of simple operation, high reaction safety performance, continuous preparation and production, and controllable and adjustable treatment capacity, is suitable for large-scale production, and is beneficial to realizing industrial application of carbon nanotubes.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
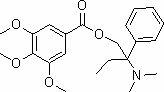
Preparation method of trimebutine
ActiveCN102276487AReduced risk of reactionLow costOrganic compound preparationAmino-hyroxy compound preparationBenzoic acidTrimebutine
The invention relates to medicine preparation methods, particularly a preparation method of trimebutine, which comprises the following steps: esterifying and amino-methylating 2-amino-2-phenylbutyric acid which is used as the raw material, and reducing into 2-(dimethylamino)-2-phenylbutyl alcohol; and synthesizing trimebutine from the 2-(dimethylamino)-2-phenylbutyl alcohol and 3,4,5-trimethoxybenzoic acid in an organic solvent by using protonic acid as a catalyst. The invention is characterized in that the esterification and amino-methylation of the 2-amino-2-phenylbutyric acid are carried out at the same time to obtain the 2-(dimethylamino)-2-phenylmethyl butyrate, and sodium borohydride is used for reduction to obtain the 2-(dimethylamino)-2-phenylbutyl alcohol. In the invention, the 2-amino-2-phenylbutyric acid is subjected to esterification and amino-methylation at the same time, and reduced into alcohol, thereby simplifying the operation, greatly reducing the production risk, enhancing the reaction yield (up to higher than 80%) and greatly lowering the raw material cost. In the last preparation step, the protonic acid is added, thereby reducing the feed amount of the 3,4,5-trimethoxybenzoic acid and lowering the reaction cost.
Owner:YUEYANG YETOP FINE CHEM
Preparation method of 5-chiorine-2-nitroaniline
InactiveCN102070466AHigh reaction yieldReduced risk of reactionOrganic compound preparationCarboxylic acid amides preparationSolvent2-Nitroaniline
The invention relates to a preparation method of 5-chiorine-2-nitroaniline. The preparation method is as follows: obtaining m-chloroacetanilide by acylation reaction of m-chloroaniline and acetylcjloride in aprotic solvent under a relative mild condition; then nitrating the m-chloroacetanilide so as to obtain 5-chiorine-2- nitracetanilide in the presence of nitric acid and anhydro; and finally, removing acetyl in the presence of Claisen alkaline so as to obtain the 5-chiorine-2-nitroaniline. By using the preparation method of the 5-chiorine-2-nitroaniline, the reaction yield is improved, the reaction danger is reduced, and the production danger is reduced, thus the safe production is ensured.
Owner:天津均凯农业科技有限公司
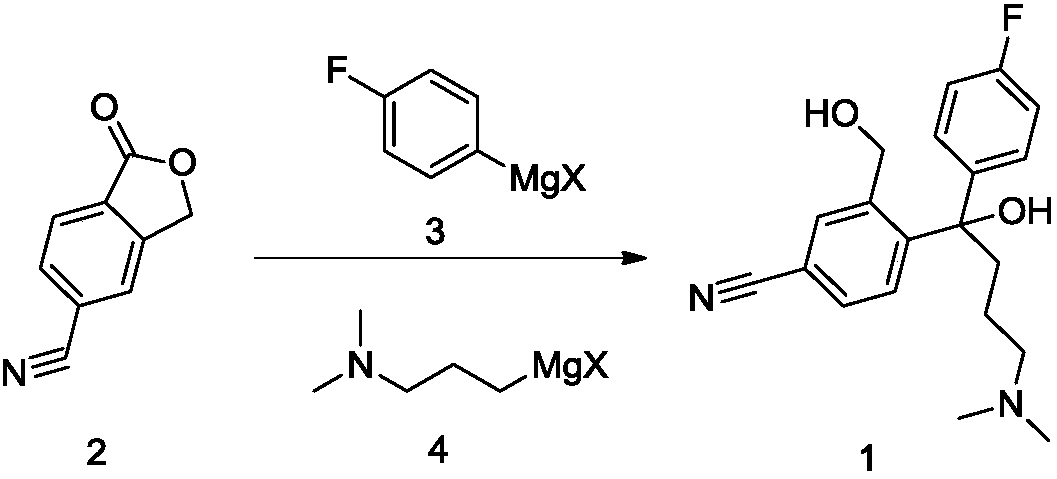
Method for continuously preparing citalopram diol
ActiveCN111302971AControl generationHigh selectivityCarboxylic acid nitrile preparationOrganic compound preparationGrignard reagentCombinatorial chemistry
The invention provides a method for continuously preparing a citalopram intermediate citalopram diol. The method comprises the following steps: preferentially mixing two Grignard reagents required bya reaction, mixing the mixed Grignard reagent with a raw material 5-cyanophthalide in a micro-mixer to obtain a reaction solution, completing the reaction of the reaction solution through a reactor toobtain a citalopram diol reaction solution, quenching, concentrating, extracting, acidifying, crystallizing, and carrying out other operations to obtain a qualified product. The citalopram diol provided by the invention is good in selectivity, high in yield, high in safety, safe, reliable, less in pollution discharge and suitable for industrial production.
Owner:SHANGHAI AOBO PHARMTECH INC LTD +1
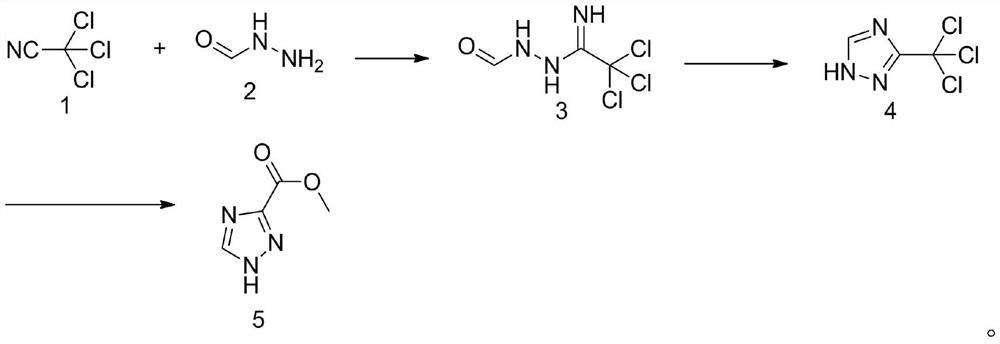
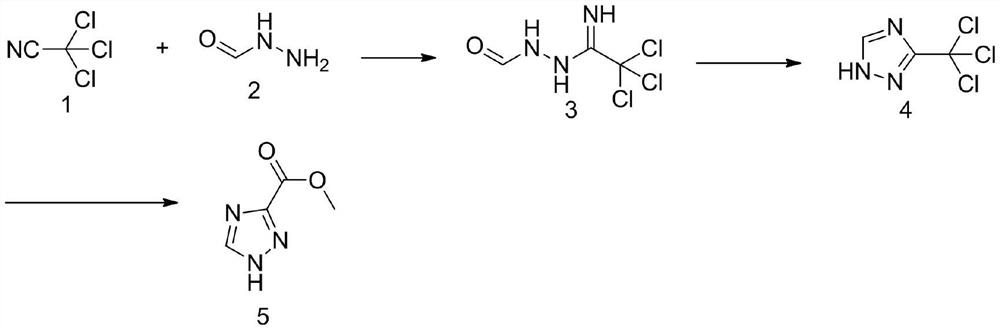
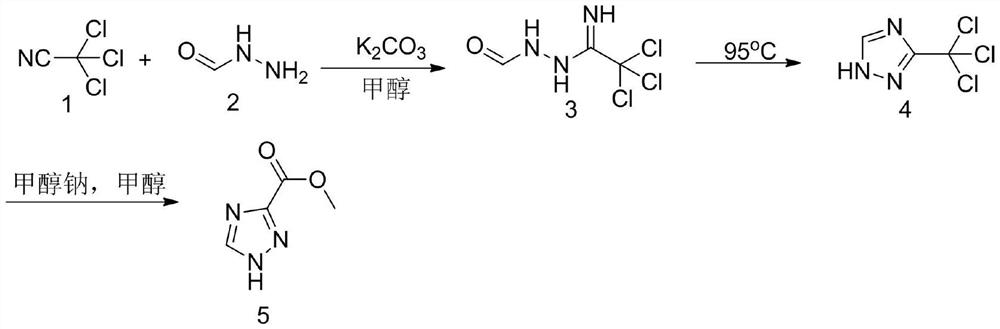
Method for synthesizing 1, 2, 4-triazole-3-carboxylic acid methyl ester
ActiveCN111808034ANovel design routeReduced risk of reactionOrganic chemistryTrichloroacetonitrileMetaclazepam
The invention discloses a method for synthesizing 1, 2, 4-triazole-3-carboxylic acid methyl ester, and belongs to the field of organic chemistry. The method comprises the following reaction steps: reacting trichloroacetonitrile 1 with formylhydrazine 2 to generate an intermediate 3, then carrying out cyclization reaction to obtain an intermediate 4, and finally carrying out alcoholysis reaction togenerate 1, 2, 4-triazole-3-carboxylic acid methyl ester 5. According to the method, only three steps of reaction are needed, the overall yield is high, dangerous diazotization deamination reaction in a traditional synthesis process is avoided, the safety risk in the reaction process is reduced, and meanwhile the production cost can be reduced.
Owner:TUOXIN GROUP +1
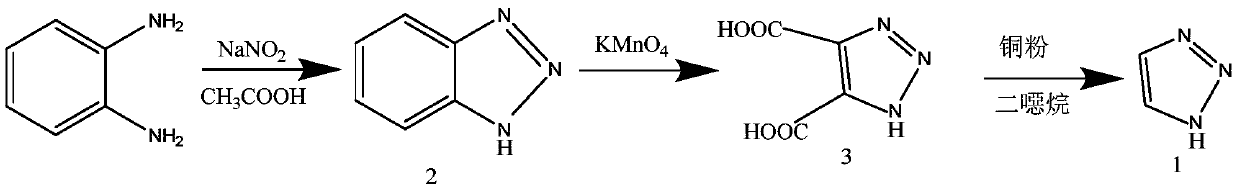
Preparation method of 1H-1, 2, 3-triazole
InactiveCN111320587ALow toxicityReduced risk of reactionOrganic chemistryPotassium permanganateTriazole
The invention provides a preparation method of 1H-1, 2, 3-triazole, which comprises the following steps: adding glyoxal into an ethanol solution of hydrazine hydrate, and carrying out reduction reaction to obtain a glyoxal dihydrazone solution; adding hydrogen peroxide into the glyoxal dihydrazone solution, carrying out a cyclization reaction to prepare a 1-amino-1, 2, 3-triazole solution; addingpotassium permanganate into the 1-amino-1, 2, 3-triazole solution, and heating to carry out a deamination reaction, so as to obtain a 1H-1, 2, 3-triazole solution. According to the method, the 1H-1, 2, 3-triazole can be prepared by a one-pot method, a solvent does not need to be added or replaced in the reaction process, nitrite and potassium permanganate which can generate solid waste are prevented from being used, and toxic substances such as toluenesulfonyl chloride and dioxane which are harmful to the environment are not used.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
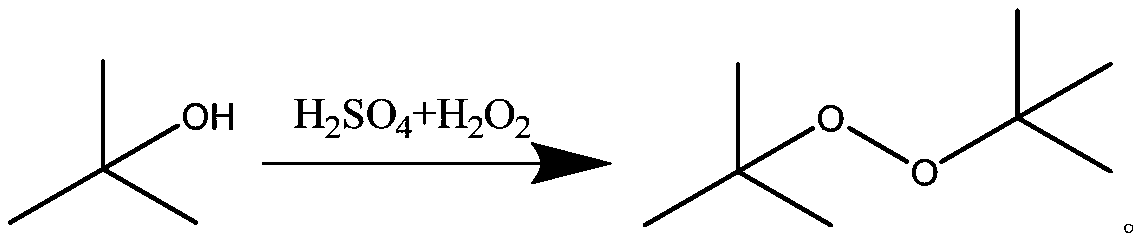
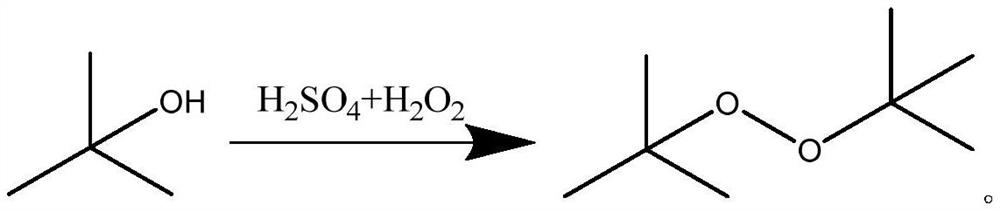
Di-tert-butyl peroxide synthesis method
ActiveCN110204472AImprove conversion rateHigh selectivityOrganic compound preparationChemical/physical/physico-chemical microreactorsChemical industryDecomposition problem
The invention belongs to the technical field of chemistry and chemical industry, and particularly relates to a di-tert-butyl peroxide synthesis method, which comprises: mixing tert-butanol and concentrated sulfuric acid in a first micro-channel continuous flow reactor, and carrying out a mixing reaction on the obtained product and hydrogen peroxide in a second micro-channel continuous flow reactor, wherein the temperature of the first micro-channel continuous flow reactor is 10-25 DEG C, the second micro-channel continuous flow reactor is formed by connecting 8-12 micro-channel mixer substrates, and the temperature of the second micro-channel continuous flow reactor is controlled at 45-65 DEG C. According to the present invention, the method has characteristics of extremely high heat transfer and mass transfer efficiency, segmental temperature control, energy consumption cost reducing and risk coefficient reducing, and can solve the product decomposition problem caused by temperature control loss and side reaction increasing; and by using the synthesis method, the reaction conversion rate is more than 99.3%, and the selectivity is more than 99.0%.
Owner:LINZIZHENGHUA ACCESSORY INGREDIENT ZIBO
Synthesis method of isopropylhydrazine
InactiveCN108047080AEmission reductionFew reaction stepsHydrazine preparationSynthesis methodsHydrazine compound
The invention provides a synthesis method of isopropylhydrazine. The synthesis method comprises the following steps: under protection of inert gas, carrying out alkylation reaction on isopropyl alcohol and hydrazine hydrate under the action of a catalyst and at a certain temperature, generating the isopropylhydrazine, separating an organic phase containing the isopropylhydrazine from a water phaseafter the reaction is finished, and then distilling the organic phase to obtain a finished product of the isopropylhydrazine; the catalyst used in reaction is sodium tripolyphosphate. Compared with the prior art, the synthesis method provided by the invention has the beneficial effects that the isopropyl alcohol and the hydrazine hydrate are adopted as raw materials to synthesize the isopropylhydrazine by a one-step method, the reaction steps are fewer, the aftertreatment is simple, the reaction dangerousness is low, the yield is high, the production cost is low, and the emission of wastes isless, so that the synthesis method is a green and environmental-friendly type production process.
Owner:重庆丽澄环保科技有限公司
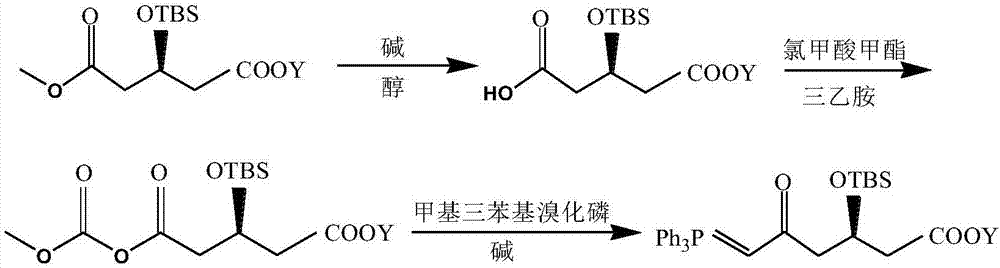
Preparation method of high-purity statin drug intermediate
InactiveCN107011378AReduce pollutionHigh ee valueGroup 5/15 element organic compoundsWaste treatmentGlutarates
The invention relates to a preparation method of a high-purity statin drug intermediate. The preparation method is characterized in that 3-hydroxyl ethyl glutarate is used as the initial raw material to prepare (3R)-tert-butyl dimethyl silyloxy-5-oxo-6-triphenyl phosphine caproate (abbreviated as J6) through substitution reaction, hydrolysis reaction, cyclization reaction, resolution reaction, hydrogenation reaction, acylation reaction and Wittig reaction. The preparation method is mild in condition, stable in process, cheap in raw material, easy in raw material obtaining, easy in three-waste treatment, low in preparation cost, high in product purity and suitable for industrial production.
Owner:南京大学淮安高新技术研究院 +1
3-N-cyclopropylmethyl-2-fluorobenzamide compound as well as preparation method and application thereof
ActiveCN112707841AEasy to synthesizeLower synthesis costBiocideOrganic compound preparationFormamidesPerylene derivatives
The invention provides a 3-N-cyclopropylmethyl-2-fluorobenzamide compound as well as a preparation method and application thereof. The compound has a structure as shown in a formula I which is described in the specification. The compound can be used for preparing an m-diamide compound with a 3-position N-cyclopropyl methyl derivative substituent, and the m-diamide compound with the 3-position N-cyclopropyl methyl derivative substituent has the characteristics of being good in fast-acting property, low in dosage and more beneficial to environmental protection when being used as an insecticide. The 3-N-cyclopropyl methyl-2-fluorobenzamide compound provided by the invention is easy to synthesize and mild in condition, and is easy to synthesize, low in synthesis cost and high in yield when being used for preparing an insecticide of the m-diamide compound with the 3-position N-cyclopropyl methyl derivative substituent.
Owner:CAC NANTONG CHEM
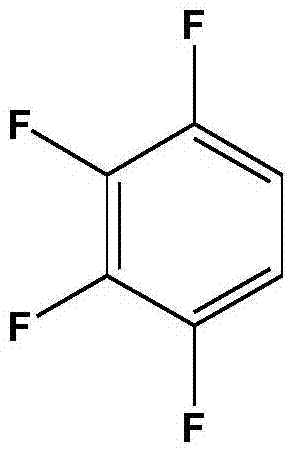
Preparation method of 1,2,3,4-tetrafluorobenzene from 2,3,4,5-tetrafluorobenzoic acid
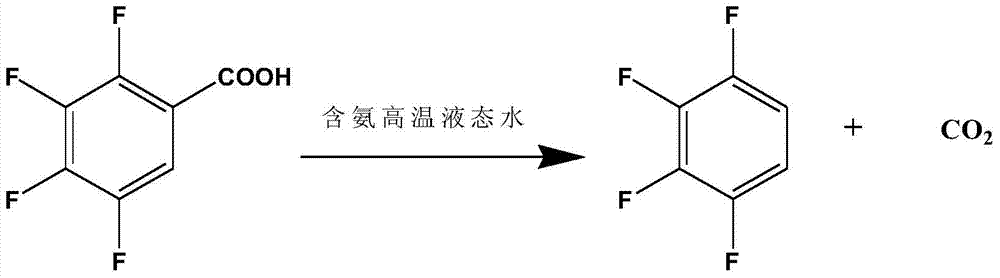
ActiveCN105330509AIncrease alkalinityFacilitated ionizationHalogenated hydrocarbon preparationReaction rateLiquid state
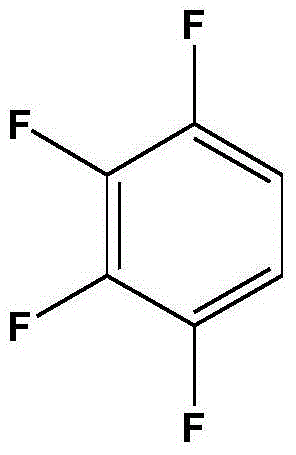
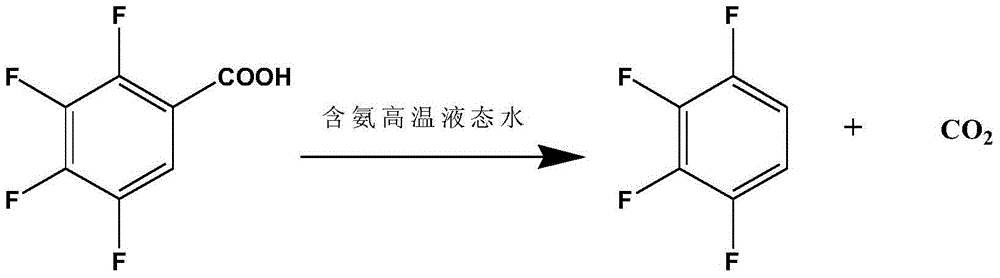
The invention discloses a preparation method of 1,2,3,4-tetrafluorobenzene from 2,3,4,5-tetrafluorobenzoic acid through decarboxylation reaction in a high temperature ammonia-containing liquid state aqueous medium at 210-260 DEG C. The preparation method has the advantages of high reaction rate, short decarboxylation reaction time, high yield, and green and environmental protection.
Owner:中化蓝天氟材料有限公司 +1
Preparation method of N-benzenesulfonyl-4-halogen-2-nitroaniline
ActiveCN114163362AReduce manufacturing difficultyMild reaction conditionsSulfonic acid amide preparationBulk chemical productionChlorobenzeneOrganic synthesis
According to the preparation method of the N-benzenesulfonyl-4-halogen-2-nitroaniline, provided by the invention, the N-benzenesulfonyl-4-halogen-2-nitroaniline can be further hydrolyzed to generate the 4-halogen-2-nitroaniline. The 4-halo-2-nitroaniline is an important organic synthesis intermediate, has a wide application prospect, and can be mainly used for preparing medicines such as maribavir, triclabendazole and the like and synthesizing various substituted benzimidazole, quinazolinone derivatives and the like. However, in the prior art, synthesis of N-benzenesulfonyl-4-halogen-2-nitroaniline has the defects of harsh reaction conditions, low process yield and relatively long reaction route. The invention relates to the technical field of synthesis of medical intermediates, which comprises the following steps: dissolving N-benzenesulfonyl aniline in 1, 2-dichloroethane, and reacting with a nitrate source and tetrabutyl ammonium halide in the presence of alkali to obtain N-benzenesulfonyl-4-halo-2-nitroaniline. The synthetic route is mild in reaction condition, simple in reaction and post-treatment process operation, low in reaction danger coefficient, low in production cost and suitable for industrial large-scale production.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Preparation method of isopropyl hydrazine
InactiveCN108285425AEmission reductionReduce process stepsHydrazine preparationAlkyl transferStrong acids
The invention provides a preparation method of isopropyl hydrazine. The method comprises the steps that under the protection of inert gas and under the action of a catalyst, alkylation reaction is conducted on isopropanol and hydrazine hydrate at a certain temperature to generate isopropyl hydrazine; an organic phase containing isopropyl hydrazine is separated from an aqueous phase after the reaction is finished; then the organic phase is rectified to obtain an isopropyl hydrazine finished product, wherein the catalyst used for the reaction is D001 macroporous strong acid cation exchange resin. Compared with the prior art, isopropanol and hydrazine hydrate serve as raw materials, isopropyl hydrazine is prepared through a one-step method, the number of process steps is small, post-treatmentis simple, the reaction risk is low, the yield is high, the production cost is low, little waste is discharged, and the method is an environment-friendly production technology.
Owner:重庆晶萃化工科技有限公司
Aldehyde synthesis method
PendingCN114835577AThree wastes lessReduced risk of reactionOrganic compound preparationCarboxylic acid esters preparationButeneBiochemical engineering
The invention discloses a synthesis method of aldehyde. The synthesis method comprises the following steps: step 1, synthesis of an intermediate 1, step 2, synthesis of an intermediate 2, and step 3, synthesis of 4-acetoxy-2-methyl-2-butene-1-aldehyde. According to the synthesis method, the problems of high production cost, more three wastes and difficulty in treatment after the 4-acetoxy-2-methyl-2-butene-1-aldehyde is synthesized by the existing technical route are solved, a large amount of three wastes are reduced, the synthesis method is more environment-friendly, and meanwhile, the reaction risk is reduced.
Owner:安徽智新生化有限公司
Preparation method for 8-amino-5-methylquinoline
InactiveCN103880743ALower requirementReduced risk of reactionOrganic chemistryQuinolineCombinatorial chemistry
The invention discloses a preparation method for 8-amino-5-methylquinoline. 8-nitro-5-methylquinoline is taken as a raw material, stannous chloride is taken as a reducing agent, a reaction is performed at room temperature, and alkalization, extraction and recrystalization are performed for obtaining 8-amino-5-methylquinoline. In the preparation method, stannous chloride is employed for replacing conventional palladium / carbon for reduction, so that requirements of the reaction on equipment is reduced, reaction dangerousness, reaction time and cost are reduced, but yield is not reduced, and the preparation method has good economic benefit.
Owner:QINGDAO VLAND BIOTECH INC
The synthetic method of di(2-ethylhexyl) peroxydicarbonate
ActiveCN110256320BImprove conversion rateHigh selectivityOrganic compound preparationChemical/physical/physico-chemical microreactorsChemical industryTemperature control
The invention belongs to the technical field of chemistry and chemical engineering, and in particular relates to a synthetic method of bis(2-ethylhexyl) peroxydicarbonate. Mix hydrogen peroxide and sodium hydroxide in the first microchannel continuous flow reactor; then mix and react with chlorinated ester in the second microchannel continuous flow reactor; the temperature of the first microchannel continuous flow reactor is 15-30°C ; The second microchannel continuous flow reactor is composed of 3-10 microchannel mixer substrate connections, and the temperature of the second microchannel continuous flow reactor is controlled to be 30-44°C. The invention has extremely high heat and mass transfer efficiency, realizes segmental temperature control, solves the problem of product decomposition caused by temperature out of control and increased side reactions, and simultaneously reduces energy consumption costs and risk factors. By adopting the synthesis method described in the invention, the reaction conversion rate is above 99.2%, and the selectivity is above 98.5%.
Owner:LINZIZHENGHUA ACCESSORY INGREDIENT ZIBO
Preparation method of 5, 5'-(perfluoropropane-2, 2-diyl) bis (2-(allyloxy) aniline)
PendingCN113636938AReduce manufacturing difficultyMild reaction conditionsOrganic compound preparationAmino-hyroxy compound preparationNitrobenzeneEngineering
The invention provides a preparation method of 5, 5'-(perfluoropropane-2, 2-diyl) bis (2-(allyloxy) aniline), and relates to the technical field of synthesis of chemical intermediates. The preparation method comprises the steps of dissolving 4, 4 '-(hexafluoroisopropylidene) diphenol in glacial acetic acid and nitric acid, reacting to obtain 2, 2-bis (3-nitro-4-hydroxyphenyl) hexafluoropropane, dissolving the product in acetonitrile, adding potassium carbonate and 3-bromopropylene, and reacting to obtain 5, 5'-(perfluoropropane-2, 2-diyl) bis (2-(allyloxy) nitrobenzene); and reacting 5, 5 '-(perfluoropropane-2, 2-diyl) bis (2-(allyloxy) nitrobenzene), iron powder and ammonium chloride to obtain 5, 5'-(perfluoropropane-2, 2-diyl) bis (2-(allyloxy) aniline). The synthetic route is mild in reaction condition, simple in reaction and post-treatment process operation, low in reaction danger coefficient, low in production cost and suitable for industrial large-scale production.
Owner:上海毕得医药科技股份有限公司
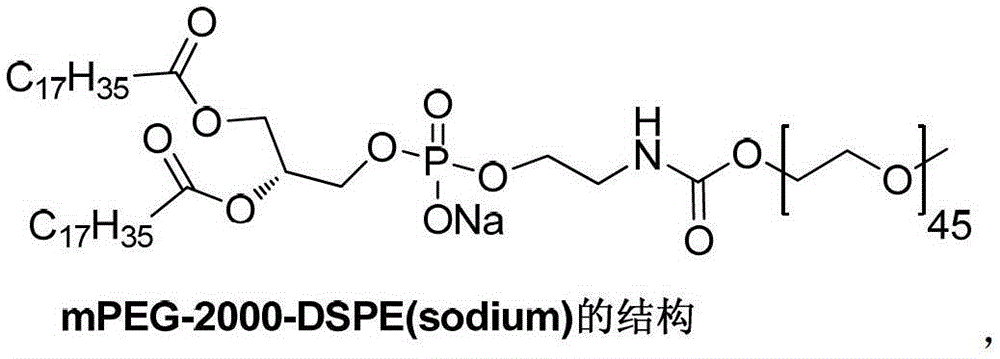
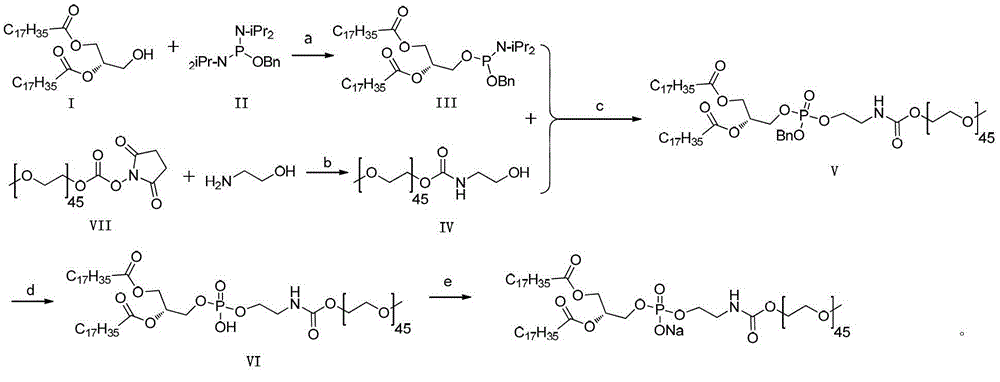
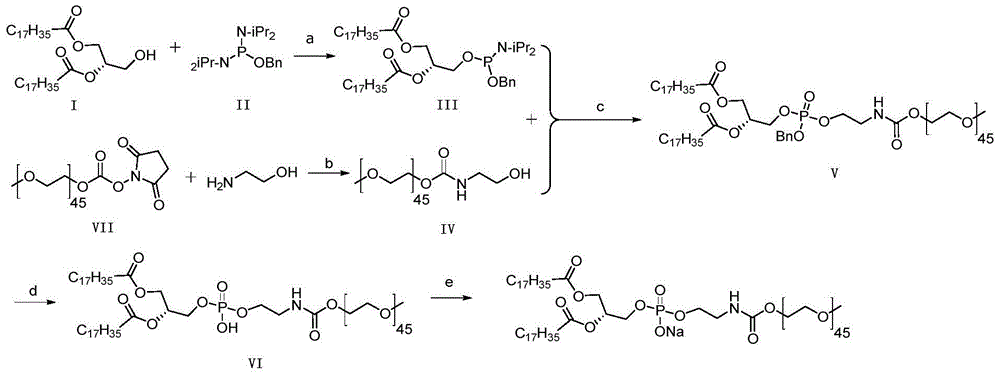
A kind of preparation method of mpeg2000-dspe sodium salt
ActiveCN103145977BReduce process stepsEasy to operatePharmaceutical non-active ingredientsActivated carbonAmmonium carbonate
The invention discloses a preparation method of mPEG2000-DSPE sodium salt, which comprises the following steps of: reacting a compound I with a compound II so as to obtain a compound III; reacting a compound VII with ethanolamine so as to obtain a compound IV; and then reacting the compound III with the compound IV so as to obtain a compound V; removing benzyl protecting groups in the compound V by using palladium on activated carbon and ammonium carbonate so as to obtain a compound VI; and finally, reacting the compound VI with alkali. The method is less in process steps, an operation of preparing the compound V by using the compound I can be completed through a one-pot reaction, so that the technological operation is simple, and an effect of industrial production is easily achieved; and a situation of taking DSPE as an intermediate is avoided, thereby reducing the production of impurities and improving the overall yield; and the reaction selectivity is high, the occurrence of side reactions and by-products is reduced, and the risk and cost of reaction are lowered.
Owner:苏州东南药业股份有限公司
Preparation method for 8-amino-7-methylquinoline
InactiveCN103880742ALower requirementReduced risk of reactionOrganic chemistryQuinolineMethyl palmoxirate
The invention discloses a preparation method for 8-amino-7-methylquinoline. 8-nitro-7-methylquinoline is taken as a raw material, stannous chloride is taken as a reducing agent, a reaction is performed at room temperature, and alkalization, extraction and recrystalization are performed for obtaining 8-amino-7-methylquinoline. In the preparation method, stannous chloride is employed for replacing conventional palladium / carbon for reduction, so that requirements of the reaction on equipment is reduced, reaction dangerousness, reaction time and cost are reduced, but yield is not reduced, and the preparation method has good economic benefit.
Owner:QINGDAO VLAND BIOTECH INC
A method for preparing 1,2,3,4-tetrafluorobenzene by 2,3,4,5-tetrafluorobenzoic acid
ActiveCN105330509BIncrease alkalinityFacilitated ionizationHalogenated hydrocarbon preparationBenzoic acidLiquid medium
The invention discloses a process for preparing 1,2,3,4-tetrafluorobenzene by decarboxylation of 2,3,4,5-tetrafluorobenzoic acid in an ammonia-containing high-temperature liquid water medium at a reaction temperature of 210-260°C. method. The method has high decarboxylation reaction rate, short decarboxylation reaction time, high yield and environmental protection.
Owner:中化蓝天氟材料有限公司 +1
System and method for testing inhibitory effect of uncontrolled reaction inhibitor
PendingCN109917081AWell mixedThe mixing subsystem uses high-pressure gas for dispersion to facilitateMaterial analysisData acquisitionPhysical chemistry
The invention discloses a system and method for testing the inhibitory effect of an uncontrolled reaction inhibitor, belongs to the technical field of testing, and relate to a system and method for testing the inhibitory effect of the uncontrolled reaction inhibitor. The system comprises a reactor subsystem, a parameter measurement subsystem, an inhibitor mixing subsystem, an inhibitor release subsystem and a data acquisition and control subsystem, wherein the inhibitor mixing subsystem and the inhibitor release subsystem are connected through a high-pressure metal hose; and the data acquisition and control subsystem is connected to the inhibitor release subsystem, the inhibitor mixing subsystem, the reactor subsystem, and the parameter measurement subsystem. The system evaluates the inhibitory effect of the uncontrolled reaction inhibitor, achieves the quantitative analysis of the inhibitory effects of different influence factors at different test levels, provides more test results ina short time, and comprehensively tests the inhibitory effect of the uncontrolled reaction inhibitor in practical engineering application so as to give the optimal uncontrolled reaction inhibition strategy.
Owner:NANJING TECH UNIV
A method for preparing 2,3,4,5-tetrafluorobenzoic acid and 1,2,3,4-tetrafluorobenzene
ActiveCN105418359BIncrease alkalinityFacilitated ionizationHalogenated hydrocarbon preparationBenzoic acidLiquid medium
The invention discloses that 2,3,4,5-tetrafluorobenzoic acid and 1,2,3 ,4‑tetrafluorobenzene method. The method has high decarboxylation reaction rate, short decarboxylation reaction time, high yield and environmental protection.
Owner:中化蓝天氟材料有限公司 +1
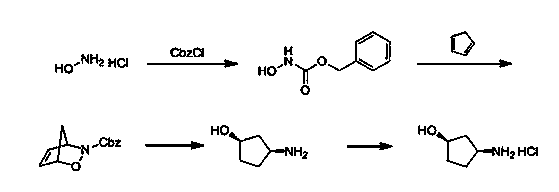
Preparation method of cis-3-amino-cyclopentanol hydrochloride
InactiveCN102633657BReduce consumptionAddress potential hazardsOrganic compound preparationAmino-hyroxy compound preparationCyclopentanolMedicinal chemistry
The invention discloses a preparation method of cis-3-amino-cyclopentanol hydrochloride, which comprises the steps of: (1) preparing a cis-3-amino-cyclopentanol intermediate A; (2) preparing a cis-3-amino-cyclopentanol intermediate B; (3) preparing a cis-3-amino-cyclopentanol crude product; (4) preparing a cis-3-amino-cyclopentanol hydrochloride crude product; and (5) obtaining the final product. The preparation method of the cis-3-amino-cyclopentanol hydrochloride has the advantages of being high in yield and purity, low in cost, safe and free from pollution.
Owner:甘肃科瑞生物科技有限公司
Synthetic method for isopropyl hydrazine
InactiveCN107986984AEmission reductionFew reaction stepsHydrazine preparationAlkyl transferHydrazine compound
The invention provides a synthetic method for isopropyl hydrazine. The method comprises the step: under the protection of inert gases, carrying out an alkylation reaction on isopropanol and hydrazinehydrate under the effect of a catalyst at a certain temperature, to generate the isopropyl hydrazine. After the reaction ends, an organic phase of isopropyl hydrazine is separated with a water phase,and then the organic phase is rectified to obtain an isopropyl hydrazine finished product, and the catalyst used for the reaction is a ZnO / Al3O3 compound catalyst. Compared with the prior art, the isopropyl hydrazine is synthesized in a one-step method by taking isopropanol and hydrazine hydrate as raw materials, and the method is few in reaction steps, simple in post-treatment, little in reactiondangerousness, high in yield, low in production cost and little in waste emission and is an environment-friendly production technology.
Owner:重庆锦杉科技有限公司
Preparation method of polycaprolactone
The invention discloses a preparation method of polycaprolactone. The preparation method comprises the following steps: adding a solvent into a raw material, hydrolyzing under an acidic or alkaline condition to form a 6-hydroxy hexanoate solution, removing water and the solvent from the 6-hydroxy hexanoate solution in a vacuum pressure environment at a temperature, carrying out 6-hydroxy hexanoate polycondensation at the same time, adjusting according to different molecular weights to obtain polycaprolactone with a molecular weight greater than 60,000, cooling the polymerization reaction materials, adding an acid to adjust the materials to be neutral, standing for layering, separating out and removing lower-layer water, washing an oil phase with water, heating after the oil phase is qualified, and performing vacuum dehydration and drying to obtain a polycaprolactone finished product. According to the method, waste generated in the production and use process of numerous caprolactone downstream products can be converted into degradable polycaprolactone products, the cost is low, recycling of the caprolactone downstream waste is achieved, environmental protection and circular economy are facilitated, and the yield of polycaprolactone is increased.
Owner:QINGDAO UNIV OF SCI & TECH
Production method of polycaprolactone
The invention discloses a production method of polycaprolactone. The production method comprises the following steps: carrying out polycondensation on 6-hydroxy hexanoate or an oligomer of 6-hydroxy hexanoate in a polycondensation temperature range and a polycondensation pressure range in the presence of a catalyst, and carrying out polycondensation for a certain time according to molecular weight requirements to prepare polycaprolactone with different molecular weights. Polycaprolactone can be obtained by direct polymerization of 6-hydroxy hexanoate or 6-hydroxy hexanoate oligomer with a catalyst, the process is simple, the cost is low, the compatibility of the product with degradable materials such as PBS, PBAT and PBT is good, the product can be widely applied to degradable solvents or additives of various biodegradable materials, the production cost is low, the polycondensation temperature range is 150-200 DEG C, the reaction temperature is low, and the whole reaction is low in risk, environment-friendly and pollution-free.
Owner:QINGDAO UNIV OF SCI & TECH
Preparation method of 4-methoxy-2-nitroaniline
ActiveCN114213261AReduce manufacturing difficultyHigh yieldOrganic compound preparationSulfonic acid amide preparationCopper nitrateNitration
The invention provides a preparation method of 4-methoxy-2-nitroaniline, and relates to the technical field of synthesis of medical intermediates, and the preparation method comprises the following steps: dissolving N-benzenesulfonyl-4-methoxyaniline in 1, 2-dichloroethane, reacting with copper nitrate trihydrate in the presence of pyridine to obtain N-benzenesulfonyl-4-methoxy-2-nitroaniline, and reacting with 1, 2-dichloroethane to obtain N-benzenesulfonyl-4-methoxy-2-nitroaniline. Then dissolving the N-benzenesulfonyl-4-methoxy-2-nitroaniline in 1, 2-dichloroethane, adding p-toluenesulfonic acid under the condition of argon protection, and carrying out a reaction so as to obtain 4-methoxy-2-nitroaniline; the synthetic route is mild in reaction condition, simple in reaction and post-treatment process operation, relatively low in reaction danger coefficient and low in production cost, reduces the environmental problems caused by the traditional nitration reaction, meets the requirements of green chemistry, has relatively high application value and relatively good economic benefits, and is suitable for industrial large-scale production.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
The synthetic method of di-tert-butyl peroxide
ActiveCN110204472BImprove conversion rateHigh selectivityOrganic compound preparationChemical/physical/physico-chemical microreactorsDi-tert-butyl peroxidePhysical chemistry
The invention belongs to the technical field of chemistry and chemical engineering, and in particular relates to a synthesis method of di-tert-butyl peroxide. Mix tert-butanol and concentrated sulfuric acid in the first microchannel continuous flow reactor; then mix and react with hydrogen peroxide in the second microchannel continuous flow reactor; the temperature of the first microchannel continuous flow reactor is 10-25°C ; The second microchannel continuous flow reactor is composed of 8-12 pieces of microchannel mixer substrates, and the temperature of the second microchannel continuous flow reactor is controlled to be 45-65°C. The invention has extremely high heat and mass transfer efficiency, realizes segmental temperature control, solves the problem of product decomposition caused by temperature out of control and increased side reactions, and simultaneously reduces energy consumption costs and risk factors. By adopting the synthesis method described in the invention, the reaction conversion rate is above 99.3%, and the selectivity is above 99.0%.
Owner:LINZIZHENGHUA ACCESSORY INGREDIENT ZIBO
Economic and safe synthesis method of zero-valent nickel coordination compound
PendingCN112159435AHigh yieldHigh purityGroup 5/15 element organic compoundsNickel organic compoundsHydration reactionMolecular sieve
The invention discloses an economic and safe synthesis method of a zero-valent nickel coordination compound, and relates to the technical field of medical catalysis. The structural formula of the zero-valent nickel coordination compound is NiRn, wherein the valence state of Ni is zero, R is a coordination ligand, n is the coordination number. The synthesis method comprises the following steps: adding tetrahydrofuran, nickel chloride hexahydrate and the coordination ligand to a reactor, stirring to react under the protection of inert gas, adding a molecular sieve, heating, stirring to react, cooling, adding zinc powder, and stirring to react; and after the reaction is finished, filtering the reaction system under the protection of inert gas, drying in vacuum to remove the organic solvent, washing, recrystallizing, and drying in vacuum to obtain the product. The method has the advantages of simple process operation, cheap and easily available raw materials, safe and stable reaction, highefficiency, high yield and high purity, and can be used for preparing several common zero-valent nickel coordination compounds.
Owner:安徽敦茂新材料科技有限公司
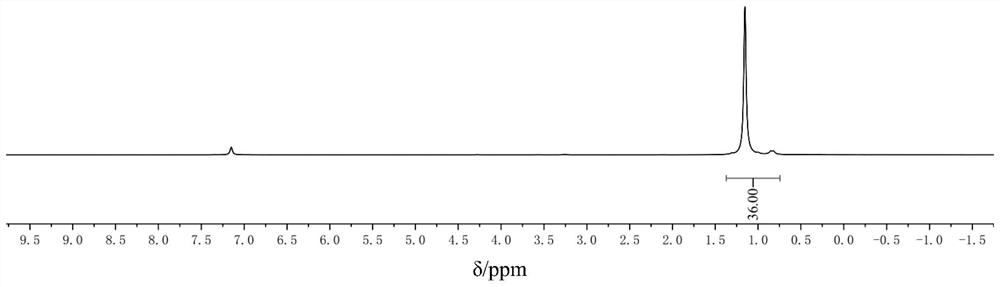
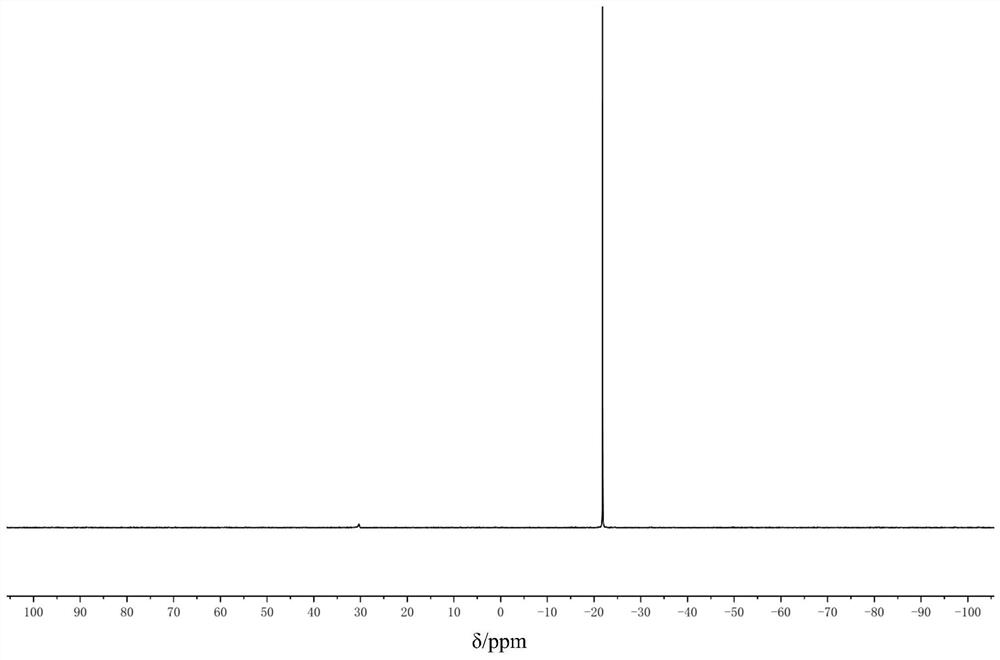
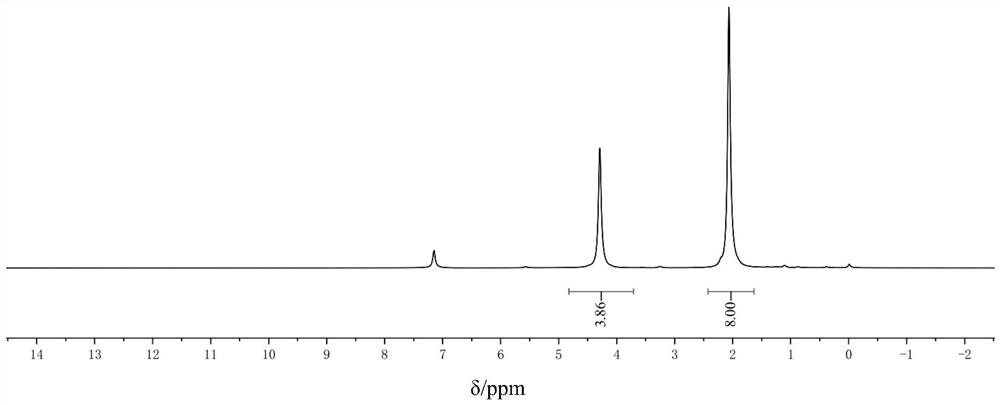
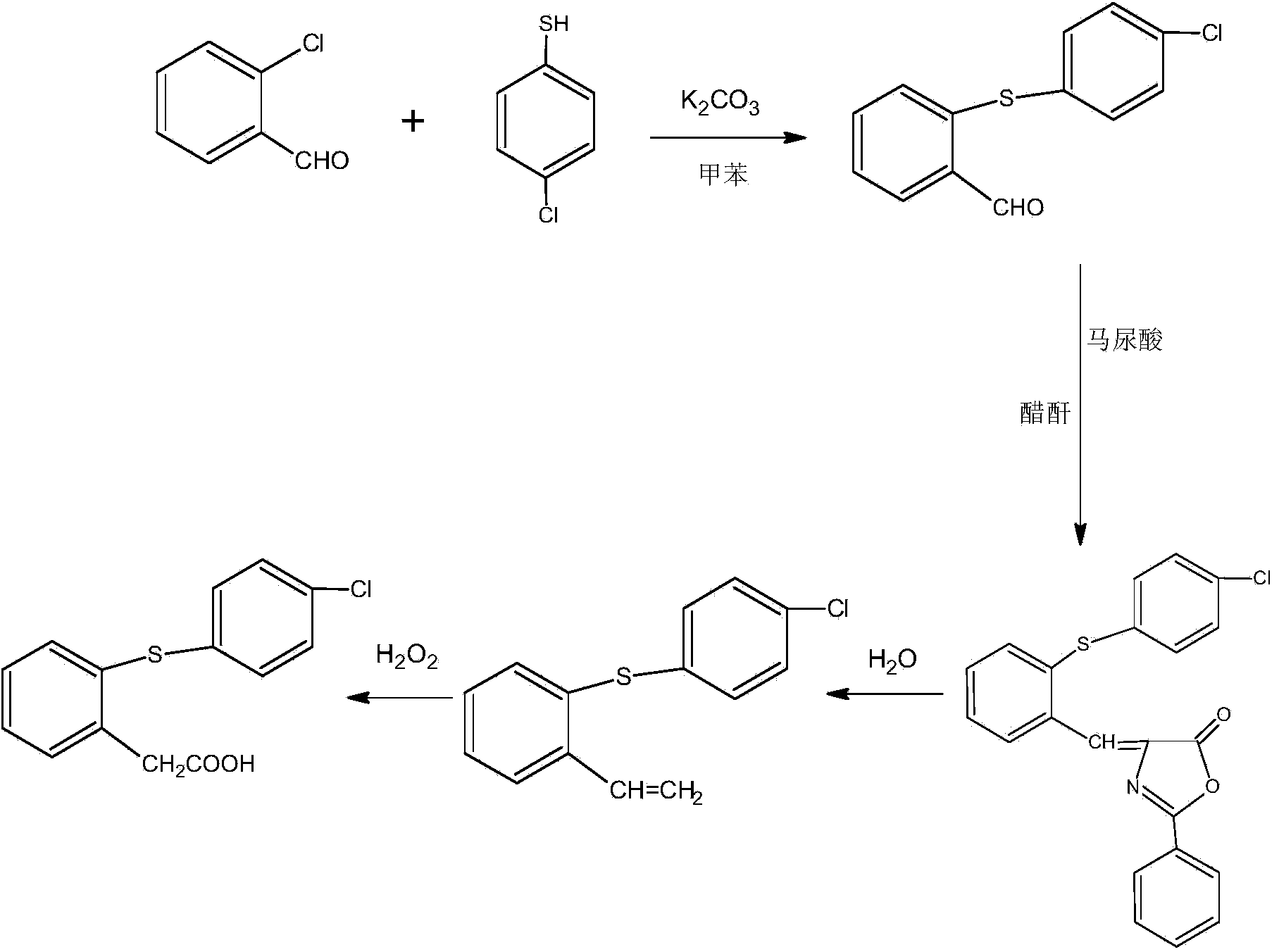
Method for preparing phenylthioacetate
The invention discloses a method for preparing phenylthioacetate. According to the method, o-chlorobenzaldehyde and p-chlorothiophenol raw materials are reacted in methylbenzene at a low temperature, so that o-chloroacetophenone serving as a raw material is abandoned; the o-chlorobenzaldehyde substitutes the o-chloroacetophenone, the methylbenzene is used as a solvent, and the condensation reaction can be performed at 50-60 DEG C, so that high-temperature condensation is not needed, the reaction is mild, the reaction hazard is reduced, three-waste pollution is reduced, the reaction yield is increased, and the cost is reduced. Hippuric acid substitutes morpholine, and the methylbenzene substitutes dioxane, so that the production cost is greatly reduced, and the reaction yield is increased. The yield of the product reaches 94%, the purity of the one-off product is more than 99%, secondary refining is not needed, and the color stability is good.
Owner:JINTAN DEPEI CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com