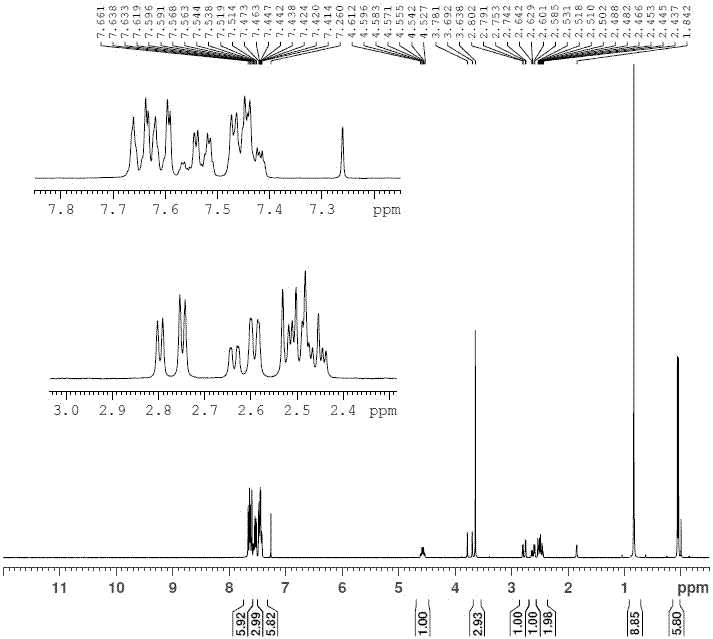
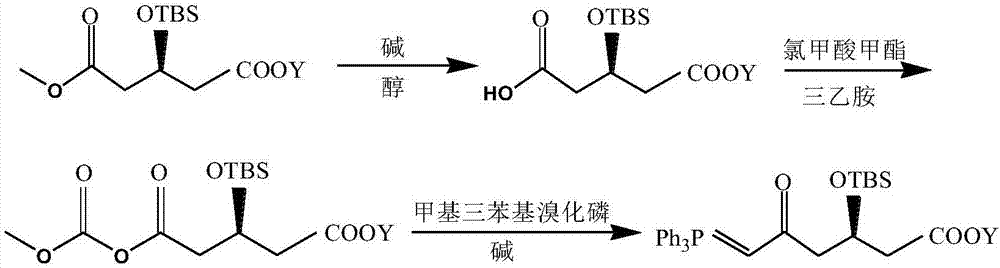
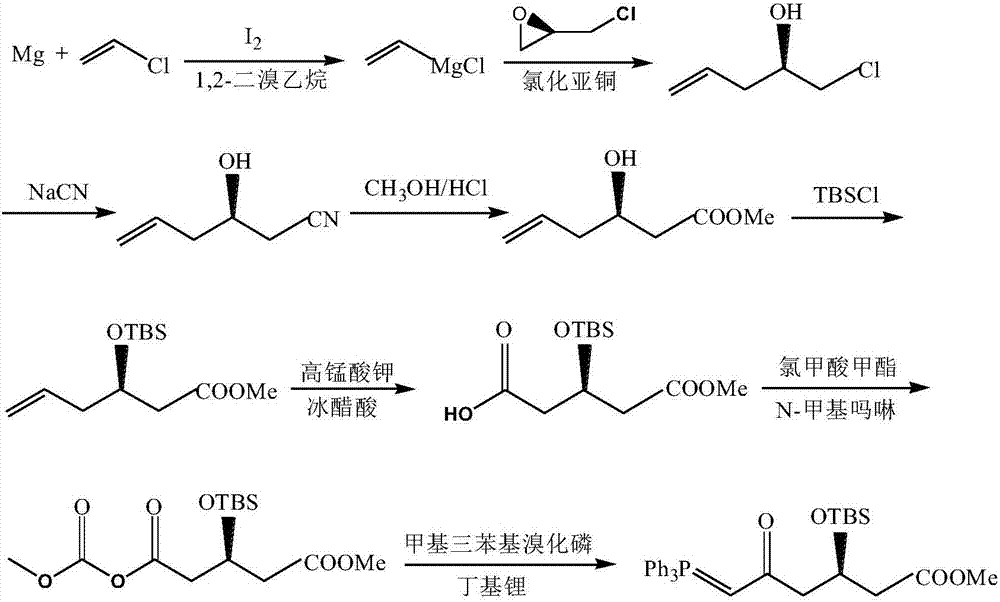
Preparation method of high-purity statin drug intermediate
A high-purity, intermediate technology, applied in the field of pharmaceutical intermediates, can solve the problems of long synthesis route, high risk and high activity, and achieve the effects of mild reaction conditions, low environmental pollution and high ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Provide the preparation method of 3-tert-butyldimethylsiloxy glutaric anhydride (J1), its steps are as follows:
[0030] (1) Add 20.4g (0.3mol) of imidazole and 75g of dichloromethane into the flask, stir and dissolve, add 20g (0.13mol) TBDMSCl, and stir for 30min; drop in 20.4g (0.1mol) of J0- 1. React at room temperature for 18 hours; wash the reaction mixture with 5 g of water, and then wash with saturated saline three times; concentrate under reduced pressure below 40°C to obtain 40 g of a light yellow liquid, namely J0-2, distill out dichloromethane and use it directly;
[0031] (2) Put 80g methanol, 12g (0.3mol) NaOH, and 1.8g water into a 200mL three-necked flask, stir to dissolve, cool down to room temperature, add dropwise a solution of J0-2 oil (0.1mol) and 16g anhydrous methanol, and keep The temperature was 20-30°C, followed by TLC, and the reaction was complete; the temperature was lowered in frozen brine, and stirred for 2 hours below 10°C; f...
Embodiment 2
[0033] Embodiment 2: Provide the preparation method of (3R)-3-tert-butyldimethylsilyloxy-glutaric acid, 1-[(R)-mandelic acid] ester (J3), the steps are as follows:
[0034] (1) Add 36g tetrahydrofuran to the three-necked flask, cool down to -25°C, add 6.7g (0.025mol, 2.5mol / L) of n-butyllithium, and keep warm for 10 minutes; continue to cool down to below -40°C, drop 5.5g (0.026mol) hexamethyldisilazane and 5g tetrahydrofuran solution, add and keep warm for 20 minutes;
[0035] (2) Continue to cool down to below -60°C, add 6.1g (0.0252mol) of benzyl mandelate and 18g of tetrahydrofuran dropwise, and control the temperature at -60°C to -90°C during the dropwise addition. To below -80°C, add J1 (4g, 0.016mol) and tetrahydrofuran 18g solution dropwise, and keep warm for 2h after dropping;
[0036] (3) add acid to neutralize, separate layers, wash with water to neutrality, and obtain J2 reaction solution;
[0037] (4) J2 reaction solution is dropped in the hydrogenation kettle, ...
Embodiment 3
[0038] Embodiment 3: Provide the preparation method of (3R)-3-tert-butyldimethylsilyloxy-glutaric acid monomethyl ester (J4), its steps are as follows:
[0039](1) Add anhydrous potassium carbonate 8g (0.058mol), anhydrous methanol 32g, N 2 Protect and lower the temperature, add J3 5g (0.0126mol), control the reaction temperature not to exceed 28°C, and stir overnight;
[0040] (2) After the reaction is completed, the reaction solution is dropped into a reaction flask equipped with 10 g of ice, 50 g of dichloromethane, and 13 g of concentrated hydrochloric acid under stirring conditions, and the pH is adjusted to 2. The layers are left to stand, and the water organic layer is washed with water, and the TLC is sampled. Determine that the mandelic acid generated by the reaction is completely removed;
[0041] (3) add 5g of anhydrous magnesium sulfate to dry, suck and filter, the filter cake is washed with dichloromethane, concentrated under reduced pressure, and evaporated to d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com