Preparation method of N-benzenesulfonyl-4-halogen-2-nitroaniline
A technology for benzenesulfonylaniline and nitroaniline, which is applied in the preparation of sulfonic acid amides, organic chemistry, etc., can solve the problems of harsh reaction conditions, long reaction routes, low process yields, etc., and achieves reduction of the reaction risk factor and reduction Production cost, the effect of simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] synthetic compound N -Benzenesulfonyl-4-bromo-2-nitroaniline
[0037] compound N- Benzenesulfonylaniline (0.2 mol), pyridine (0.3 mol), copper nitrate trihydrate (0.3 mol), tetrabutylammonium bromide (0.4 mol) were added to 200 mL of 1,2-dichloroethane, After the raw materials are dissolved, the reaction system is heated to 95°C-105°C, generally 100°C, and the reaction process is monitored by LCMS and HPLC. When the reaction time reaches 12h, it is processed. The reaction solution was poured into 1 times the volume of ice water, and the pH was adjusted to pH = 6. A large amount of solids precipitated, extracted three times with 100 mL of 1,2-dichloroethane each time, combined the organic phases, dried over anhydrous sodium sulfate, and filtered. 1,2-Dichloroethane to wash the filter cake. The filtrate was removed in vacuo to give 54.3 g of compound N -Benzenesulfonyl-4-bromo-2-nitroaniline, yield 76%, HPLC purity 95%.
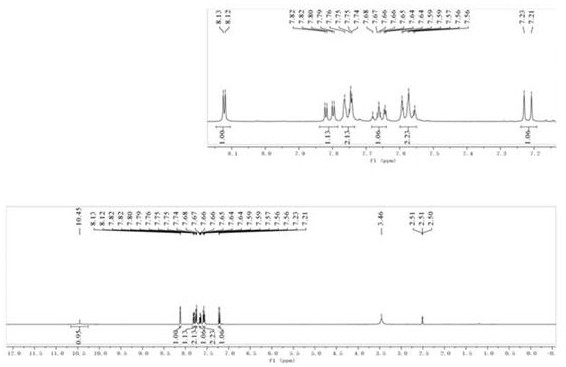
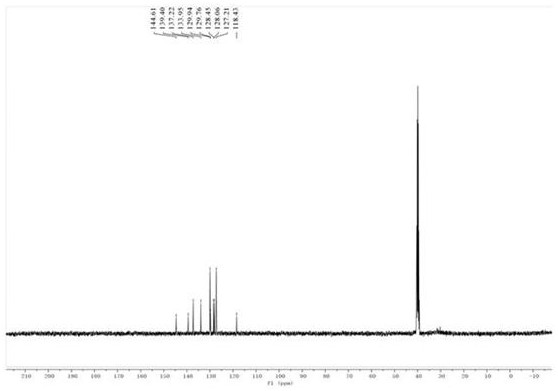
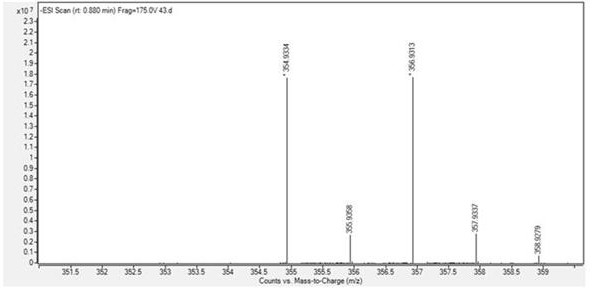
[0038] compound N The H NMR spectrum of -ben...
Embodiment 2
[0045] For embodiment 2 and embodiment 3 embodiment 1, changed the consumption of copper nitrate trihydrate; Wherein embodiment 3 then reduced the consumption of copper nitrate trihydrate, and reaction yield was reduced. Embodiment 4 increases the consumption of copper nitrate trihydrate, and the resulting reaction yield and embodiment 1 reduce slightly.
[0046] Compared with Example 1, Embodiment 4 reduces the consumption of pyridine, slows down the reaction rate of this step reaction, and makes the reaction yield decrease to some extent. For Example 5 and Example 11, the amount of pyridine was increased, so that the reaction yield did not rise.
[0047] Compared with embodiment 1, embodiment 6 to embodiment 8 replace pyridine with different bases; compared with pyridine, potassium acetate, potassium tert-butoxide, and cesium carbonate all promote the generation of side reactions, which also reduce the reaction yield. Rate.
[0048] Compared with embodiment 1, embodiment 9...
Embodiment 11
[0050] synthetic compound N -Benzenesulfonyl-4,5-dichloro-2-nitroaniline
[0051] compound N -Benzenesulfonyl-3-chloroaniline (0.2 mol), potassium acetate (0.3 mol), copper nitrate trihydrate (0.3 mol), tetrabutylammonium chloride (0.4 mol) were added to 200 mL of 1,2-di In ethyl chloride, after the raw materials are dissolved, the reaction system is heated to 95°C-105°C, generally 100°C, and the reaction process is monitored by LCMS and HPLC. When the reaction time reaches 12 h, it is processed. The reaction solution was poured into 1 times the volume of ice water, and the pH was adjusted to pH = 6. A large amount of solids precipitated, extracted three times with 100 mL 1,2-dichloroethane each time, combined the organic phases, dried over anhydrous sodium sulfate, and filtered. 1,2-Dichloroethane to wash the filter cake. The filtrate was removed in vacuo to give 44.4 g of compound N -Benzenesulfonyl-4-chloro-2-nitroaniline, yield 71%, HPLC purity 95%.
[0052] compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com