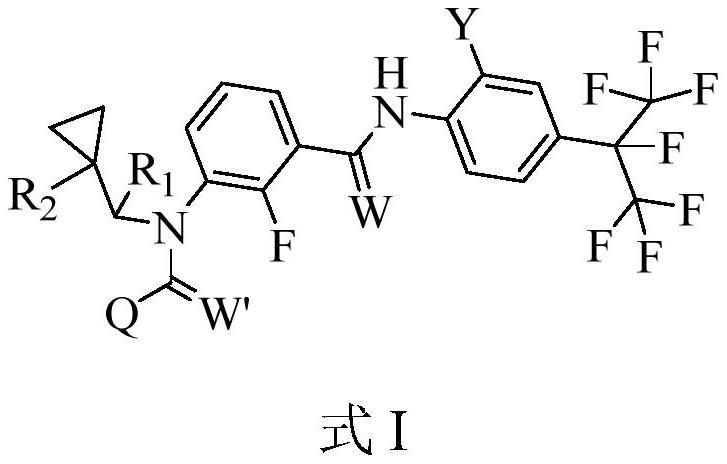
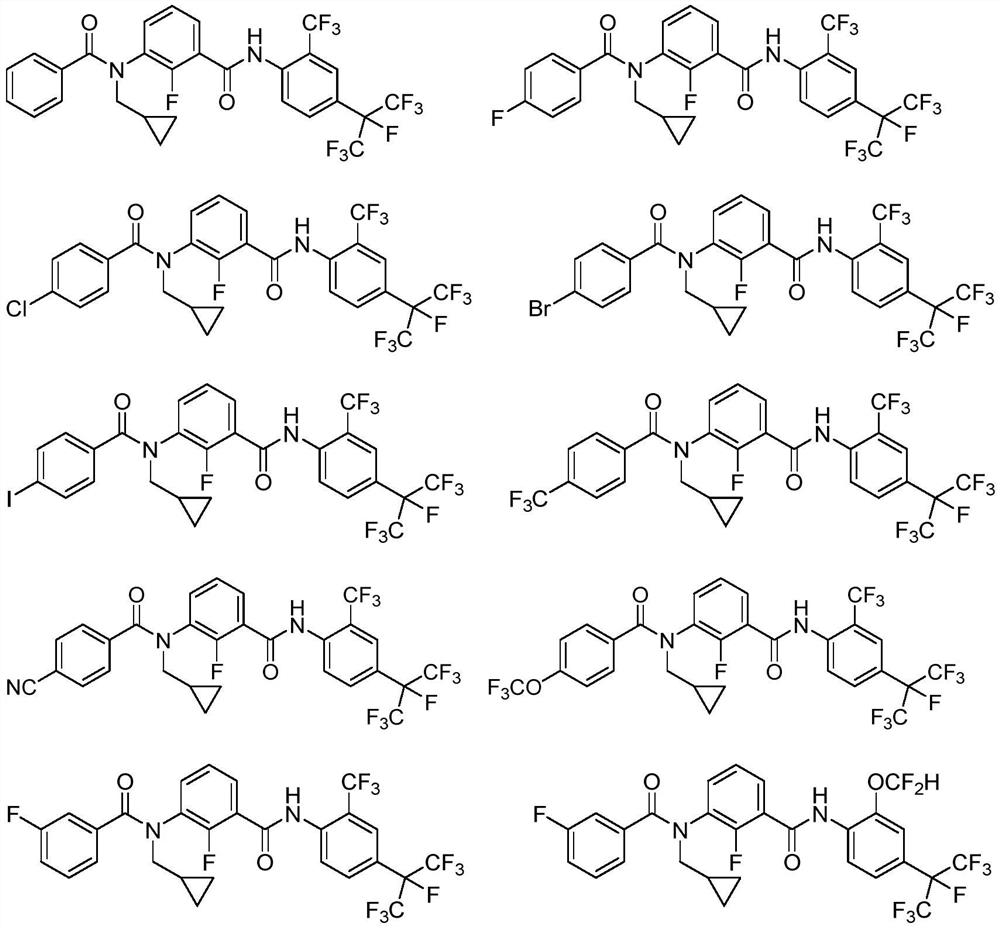
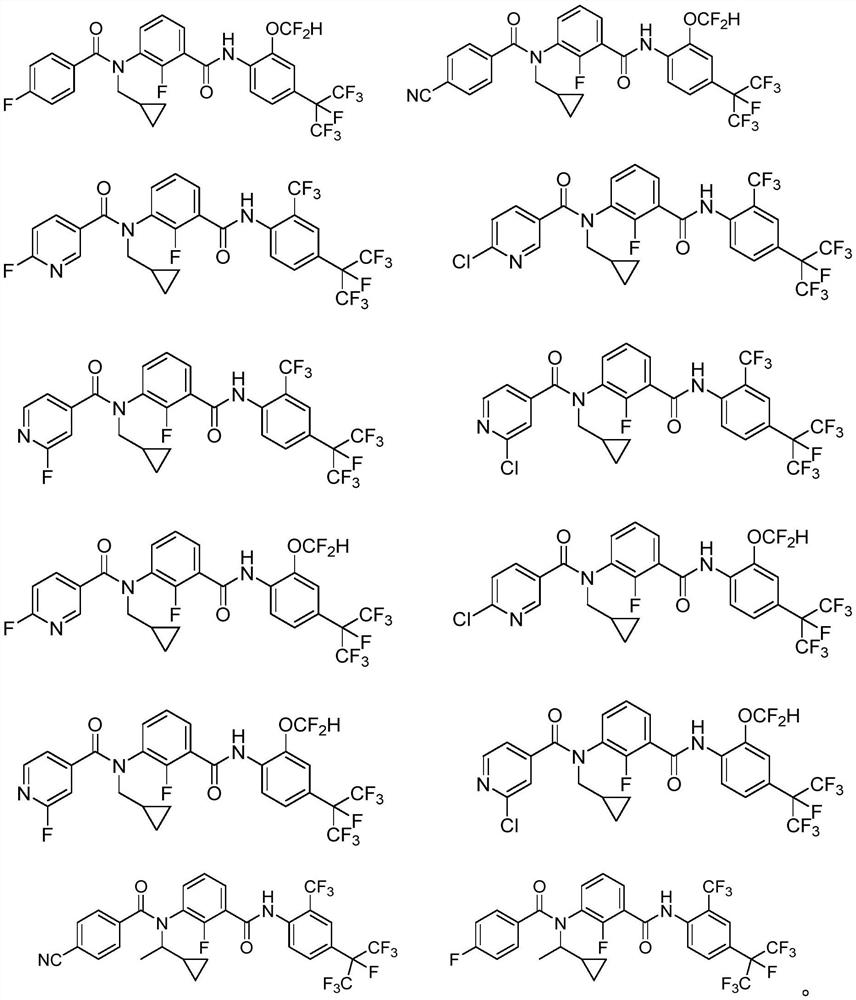
3-N-cyclopropylmethyl-2-fluorobenzamide compound as well as preparation method and application thereof
A technology of fluorobenzamide and cyclopropylmethyl, which is applied in the field of 3-N-cyclopropylmethyl-2-fluorobenzamide compounds and their preparation, and can solve the problems of low yield and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0125] The technical solutions of the present invention will be further described below through specific embodiments. It should be clear to those skilled in the art that the embodiments are only for helping to understand the present invention, and should not be regarded as specific limitations on the present invention.
[0126] Synthesis Example 1
[0127] 3-[N-cyclopropylmethyl)]-2-fluoro-N-[2-trifluoromethyl-4-(1,1,1,2,3,3,3-heptafluoropropan-2-yl ) phenyl] benzamide (compound number 2.1) preparation, the preparation method is as follows:
[0128](1) N-[2-trifluoromethyl-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-phenyl]-2-fluoro-3-nitro Synthesis of phenylbenzamides
[0129]
[0130] Add 2-fluoro-3-nitrobenzoic acid (5.9g, 31.9mmol), toluene (60mL), and thionyl chloride (7.6g, 63.8mmol) successively to the reaction flask, stir the reaction under reflux for 2h, reduce Concentrate under reduced pressure to obtain 2-fluoro-3-nitrobenzoyl chloride. 2-Trifluoromethyl-4-(1,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com