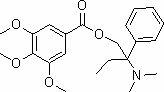
Preparation method of trimebutine
A technology of amino and dimethylamino, which is applied in the field of pharmaceutical preparation, achieves good recovery rate, reduces production cost, and is beneficial to centralized treatment and recovery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In a 2L four-necked bottle, add 150g of 30% sodium hydroxide aqueous solution, add 180g of 2-amino-2-phenylbutyric acid under stirring, stir for 1 hour, add 30% sodium hydroxide aqueous solution in an appropriate amount, control the pH=10, reduce Distilled water until viscous, cooled, filtered, and dried to obtain 200 g of 2-amino-2-phenylbutyric acid sodium salt.
[0033] Add 200g of 2-amino-2-phenylbutyric acid sodium salt and 500ml of xylene to a 2L four-neck flask, heat to 110°C, add 300g of dimethyl sulfate dropwise, keep stirring for 2 hours after the dropwise addition, and cool to 60± 2°C, add water, stir and cool to room temperature. Separate the layers, remove the water layer, recover the solvent under reduced pressure, and then rectify under high vacuum (2~3mmHg) to obtain the product to obtain 195g of methyl 2-(dimethylamino)-2-phenylbutyrate, with a yield of 88%.
[0034] In a 500ml four-necked bottle, slowly add 25g of anhydrous zinc chloride to 100ml of ...
Embodiment 2
[0037] In a 2L four-necked bottle, add 150g of 30% sodium hydroxide aqueous solution, add 180g of 2-amino-2-phenylbutyric acid under stirring, stir for 1 hour, add 30% sodium hydroxide aqueous solution in an appropriate amount, control the pH=10, reduce Distilled water until viscous, cooled, filtered, and dried to obtain 200 g of 2-amino-2-phenylbutyric acid sodium salt.
[0038] Add 200g 2-amino-2-phenylbutyric acid sodium salt and 600ml toluene to a 2L four-necked flask, heat to reflux, add 300g dimethyl sulfate dropwise, keep stirring for 2 hours after the dropwise addition, and cool to 60±2°C , add water, stir and cool to room temperature. Separate the layers, remove the water layer, recover the solvent under reduced pressure, and then rectify the product under high vacuum (2~3mmHg) to obtain 199g of methyl 2-(dimethylamino)-2-phenylbutyrate, with a yield of 90%.
[0039] In a 500ml four-necked bottle, slowly add 25g of anhydrous aluminum trichloride to 100ml of ethylene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com