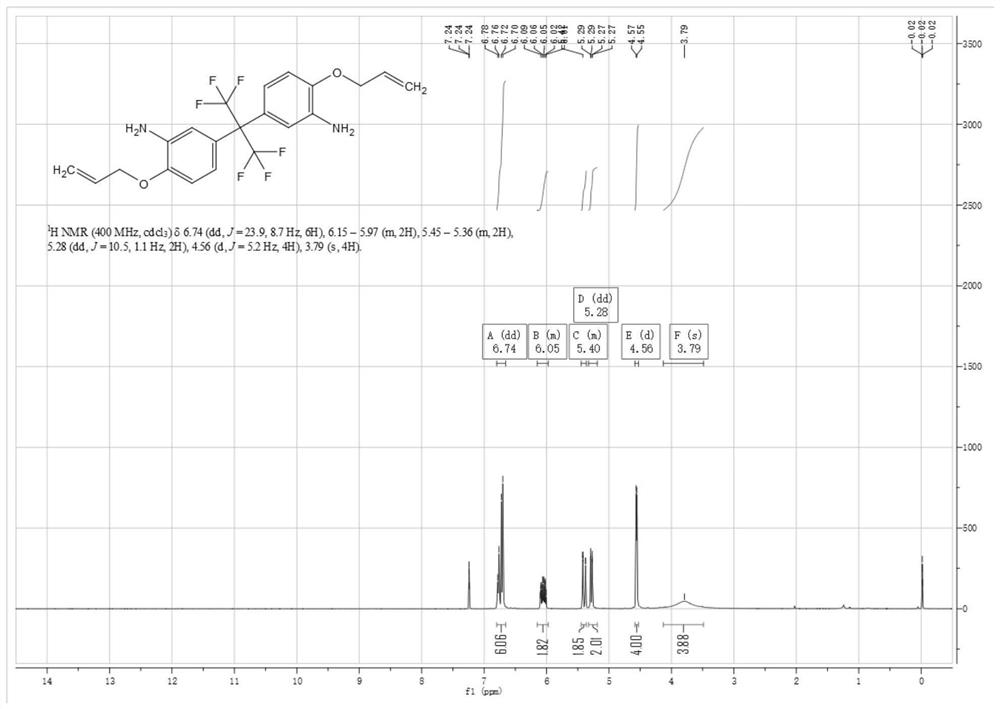
Preparation method of 5, 5'-(perfluoropropane-2, 2-diyl) bis (2-(allyloxy) aniline)
A technology of perfluoropropane and allyloxy, which is applied in the field of preparation of 5,5'-bisaniline), can solve the problems of incapable of industrialized production, severe reaction conditions, low process yield and the like, and reduces the reaction risk factor. , The effect of reducing production cost and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
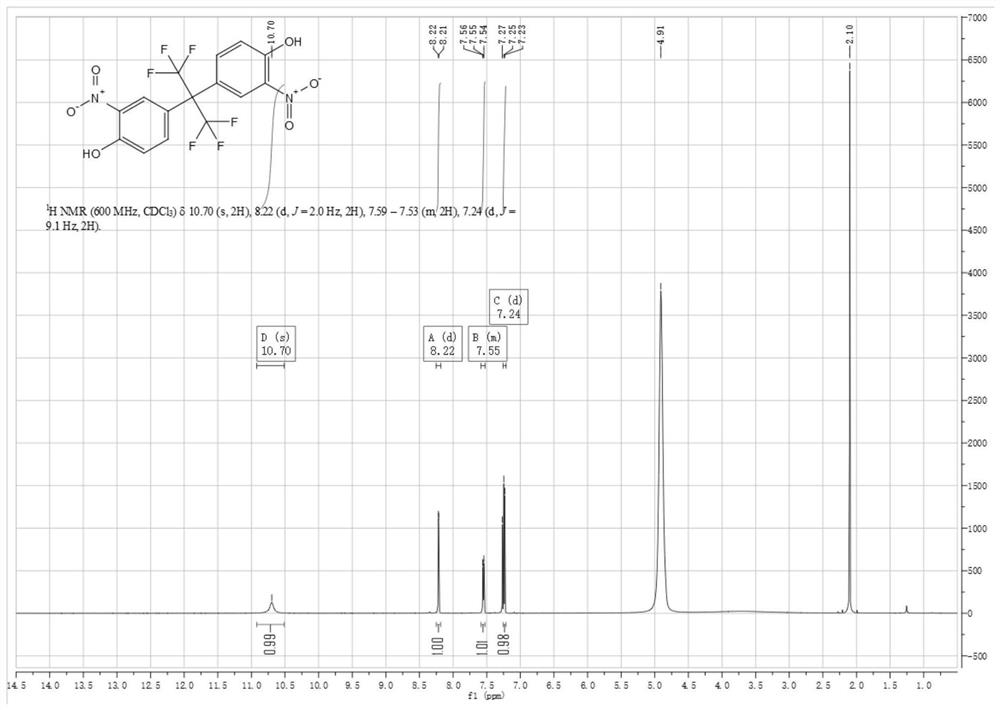
[0042] Synthesis of compound (2) 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane in the first step
[0043] Compound (1) 4,4'-(hexafluoroisopropylidene)diphenol (130g, 0.387mol, 1eq) was added to 930mL of glacial acetic acid (975g, 16.24mol, 42eq), after the raw material was dissolved, the The reaction system was heated to 28°C-29°C, generally 29°C, which is the temperature at which the pilot reaction was initiated, and nitric acid (85.3g, 1.353mol, 3.5eq) was added. In order to reduce the risk factor of the reaction and initiate the reaction first, the dropping process of nitric acid includes at least two stages; in the first stage, 5%-10% of the total amount of nitric acid is first added dropwise to the solution to initiate the reaction, that is, about 9g is added dropwise. When nitric acid is used, the temperature of the reaction solution starts to rise slowly, and no longer rises after reaching 32°C. Then, in the second stage, the remaining nitric acid is continued to be...
example 2
[0054] Synthesis of compound (2) 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane in the first step
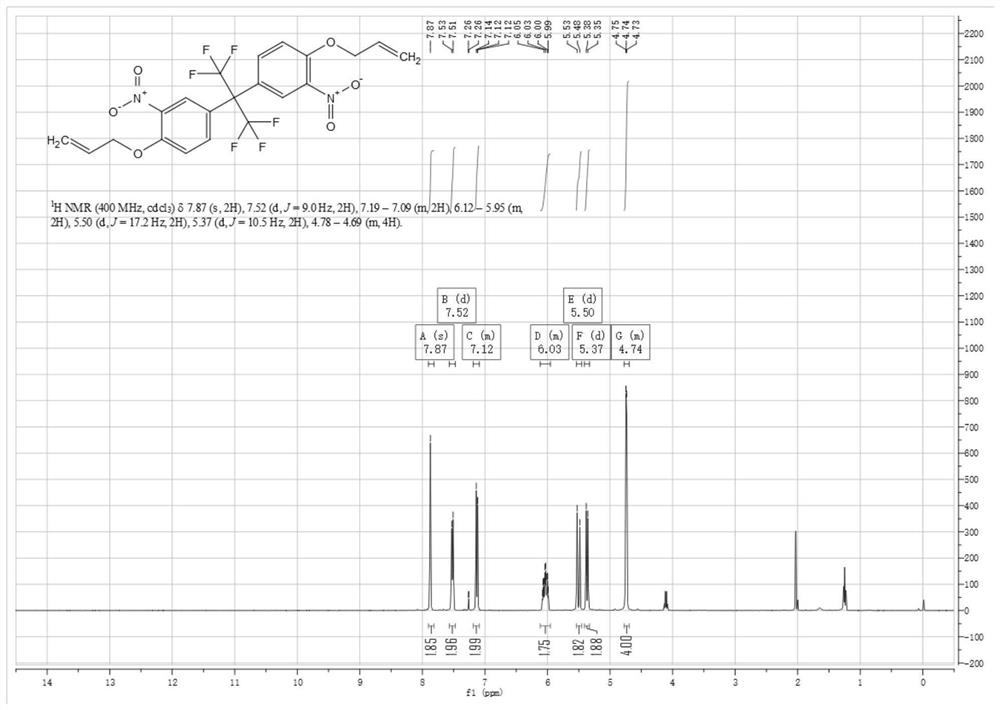
[0055] Compound (1) 4,4'-(hexafluoroisopropylidene)diphenol (130g, 0.387mol, 1eq) was added to 930mL of glacial acetic acid (975g, 16.24mol, 42eq), after the raw material was dissolved, the The reaction system was heated to 29° C., and nitric acid (48.7 g, 0.773 mol, 2.0 eq) was added in batches. When the first batch of about 4.87g was added dropwise, the temperature of the reaction solution began to rise slowly, and the temperature stopped rising after reaching 32°C. The second batch of nitric acid continued to be added dropwise, and the temperature of the reaction solution rose slightly during the dropwise addition, up to 34°C. After the dropwise addition, the reaction process was monitored by LCMS and HPLC, and the reaction was carried out when the reaction time was about 2.5 hours. The reaction solution was poured into 2 times the volume of ice water, a large amount of s...
Embodiment 3
[0061] The difference between embodiment 3, embodiment 4 and embodiment 1 is only that compound (1) 4,4'-(hexafluoroisopropylidene) diphenol and nitric acid react with different substance ratios; embodiment 5, embodiment The difference between 6 and Example 1 is only that compound (2) 2,2-bis(3-nitro-4-hydroxyphenyl) hexafluoropropane reacts with 3-bromopropene in different molar ratios; embodiment 7, The only difference between Example 8 and Example 1 is that compound (3) 5,5'-(perfluoropropane-2,2-diyl)bis(2-(allyloxy)nitrobenzene) and ammonium chloride The molar ratio of the reacted substances is different; the difference between embodiment 9, embodiment 10 and embodiment 1 is only that compound (3) 5,5'-(perfluoropropane-2,2-diyl)bis(2-(ene Propoxy) nitrobenzene) reacts with iron powder in different molar ratios; the target products obtained in each embodiment are shown in Table 1 below.
[0062] Table 1 prepares the reaction conditions and product yield of compound (4) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com