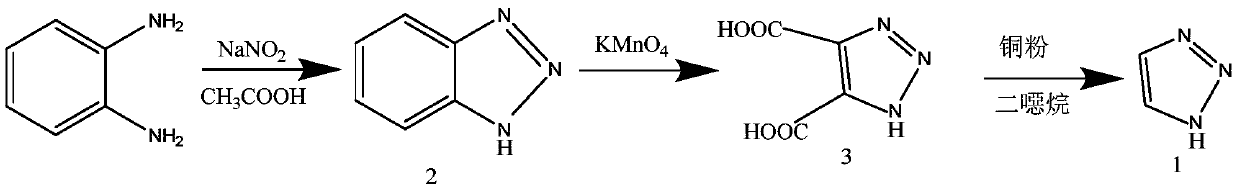
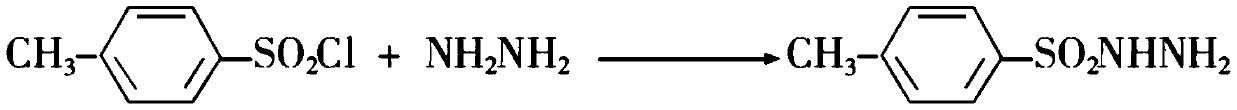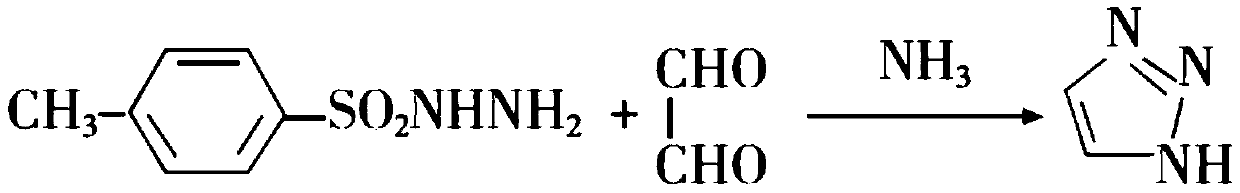Preparation method of 1H-1, 2, 3-triazole
A 1H-1, triazole technology, applied in the direction of organic chemistry and the like, can solve the problems of high price, cumbersome process, unfriendly environment, etc., and achieve the effect of being beneficial to industrial production, simplifying the synthesis route, and cleaning the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] 1H-1,2,3-triazole C provided according to the present invention 2 h 3 N 3 The preparation method comprises the following steps:
[0026] In hydrazine hydrate N 2 h 4 ·H 2 O ethanol C 2 h 6 Add glyoxal C to the O solution 2 h 2 o 2 , carry out the reduction reaction to obtain glyoxal dihydrazone C 2 h 6 N 4 solution;
[0027] To the glyoxal dihydrazone C 2 h 6 N 4 The solution was added to hydrogen peroxide H 2 o 2 , carry out the cyclization reaction to obtain 1-amino-1,2,3-triazole C 2 h 4 N 4 solution;
[0028] To the 1-amino-1,2,3-triazole C 2 h 4 N 4 Add potassium permanganate KMnO to the solution 4 , heated for deamination reaction to obtain 1H-1,2,3-triazole C 2 h 3 N 3 solution.
[0029] Specifically, the reaction process according to the preparation method of the present invention is shown as follows:
[0030]
[0031] In the reduction reaction step, hydrazine hydrate N 2 h 4 ·H 2 O can adopt the hydrazine hydrate aqueous solu...
Embodiment 1
[0044] Add hydrazine hydrate N to the 2000ml reaction bottle 2 h 4 ·H 2 O (80%) 125.0g (2mol), ethanol C 2 h 6 O304.71g (6.61mol), add dropwise glyoxal C at 0°C 2 h 2 o 2 (40%) 145.1g (1mol), the dropping time is 60min, carries out reduction reaction 2 hours, makes glyoxal dihydrazone solution;
[0045] Add hydrogen peroxide H dropwise to the above-mentioned glyoxal dihydrazone solution 2 o 2 (30%) 147.3g (1.3mol), the dropping time is 180min, and the cyclization reaction is carried out at 5°C for 180min to obtain a 1-amino-1,2,3-triazole solution;
[0046] Add potassium permanganate KMnO to the above 1-amino-1,2,3-triazole solution 4 252.8g (1.6mol), react at 25°C for 35min to obtain 1H-1,2,3-triazole solution;
[0047] To the above 1H-1,2,3-triazole solution was added dichloromethane CH 2 Cl 2 170g (2mol) was extracted, the solvent was spin-dried, and the product was distilled at 76°C and a vacuum of 1.03KPa to obtain 48.35g of the product. Yield 70%.
Embodiment 2
[0049] Add hydrazine hydrate N to the 2000ml reaction bottle 2 h 4 ·H 2 O (80%) 187.5g (3mol), ethanol C 2 h 6 O478.83g (10.41mol), glyoxal C was added dropwise at 0°C 2 h 2 o 2 (40%) 217.65g (1.5mol), the dropping time is 120min, carries out reduction reaction 3 hours, makes glyoxal dihydrazone solution;
[0050] Add hydrogen peroxide H dropwise to the above-mentioned glyoxal dihydrazone solution 2 o 2 (30%) 238.1g (2.1mol), the dropwise addition time is 160min, and the cyclization reaction is carried out at 45°C for 120min to obtain a 1-amino-1,2,3-triazole solution;
[0051] Add potassium permanganate KMnO to the above 1-amino-1,2,3-triazole solution 4 316.2g (2mol), react at 15°C for 27min to obtain 1H-1,2,3-triazole solution;
[0052] To the above 1H-1,2,3-triazole solution was added dichloromethane CH 2 Cl 2 229.5g (2.7mol) was extracted, the solvent was spin-dried, and the product was distilled at 78°C and a vacuum of 1.03KPa to obtain 49.73g of the produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com