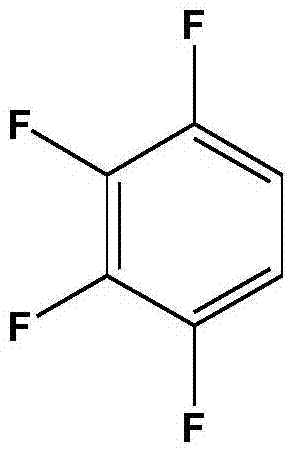
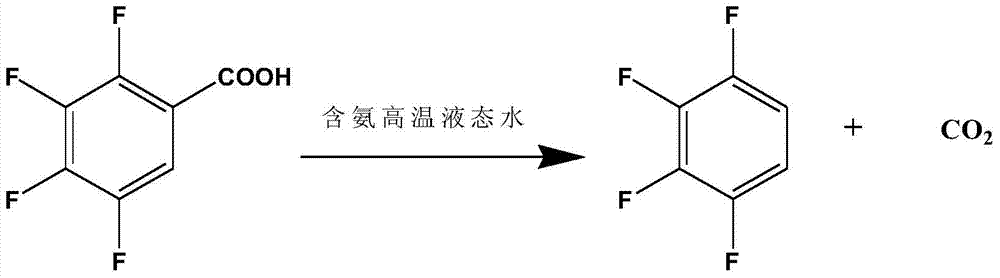
A method for preparing 1,2,3,4-tetrafluorobenzene by 2,3,4,5-tetrafluorobenzoic acid
A technology of tetrafluorobenzoic acid and tetrafluorophthalic acid, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry and other directions, can solve problems such as high toxicity and environmental pollution, and achieves improved alkalinity and reduced reaction. Dangerousness, the effect of reducing the side reaction of fluorine atom substitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 300g of deionized water and 37.5g of 2,3,4,5-tetrafluorobenzoic acid into a 500mL intermittent autoclave, start stirring, and heat up to 240°C for 4 hours of decarboxylation reaction; after the decarboxylation reaction is completed, open the exhaust valve to relieve pressure , cooled to room temperature, after standing, the liquid-liquid layer was separated to obtain an organic phase and an aqueous phase, and the organic phase was rectified to obtain 14.5 g of 1,2,3,4-tetrafluorobenzene, and the purity of the product was 98.1% through HPLC analysis. The yield was 50.0%.
Embodiment 2
[0027] Add 300g of ammonia solution with an ammonia concentration of 0.5g / L and 42.8g of 2,3,4,5-tetrafluorobenzoic acid into a 500mL batch-type high-pressure reactor, start stirring, and heat up to 210°C for 5 hours of decarboxylation reaction; after the decarboxylation reaction is completed , open the exhaust valve to relieve the pressure, and recover the ammonia in the kettle; cool down to room temperature, and after standing still, the liquid-liquid layer is separated to obtain an organic phase and an aqueous phase, and the organic phase is rectified to obtain 1,2,3,4-four 25.3 g of fluorobenzene, the purity of the product analyzed by HPLC was 98.5%, and the yield was 76.4%.
Embodiment 3
[0029] Add 300g of ammonia solution with an ammonia concentration of 1g / L and 50.0g of 2,3,4,5-tetrafluorobenzoic acid into a 500mL intermittent high-pressure reactor, start stirring, and heat up to 220°C for 4.5 hours of decarboxylation reaction; after decarboxylation is completed, Open the exhaust valve to relieve the pressure, recover the ammonia in the kettle; cool down to room temperature, and after standing still, the liquid-liquid layer is separated to obtain an organic phase and an aqueous phase, and the organic phase is rectified to obtain 1,2,3,4-tetrafluoro 32.5 g of benzene, the purity of the product analyzed by HPLC was 98.5%, and the yield was 84.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com