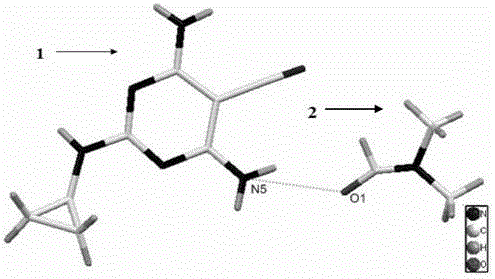
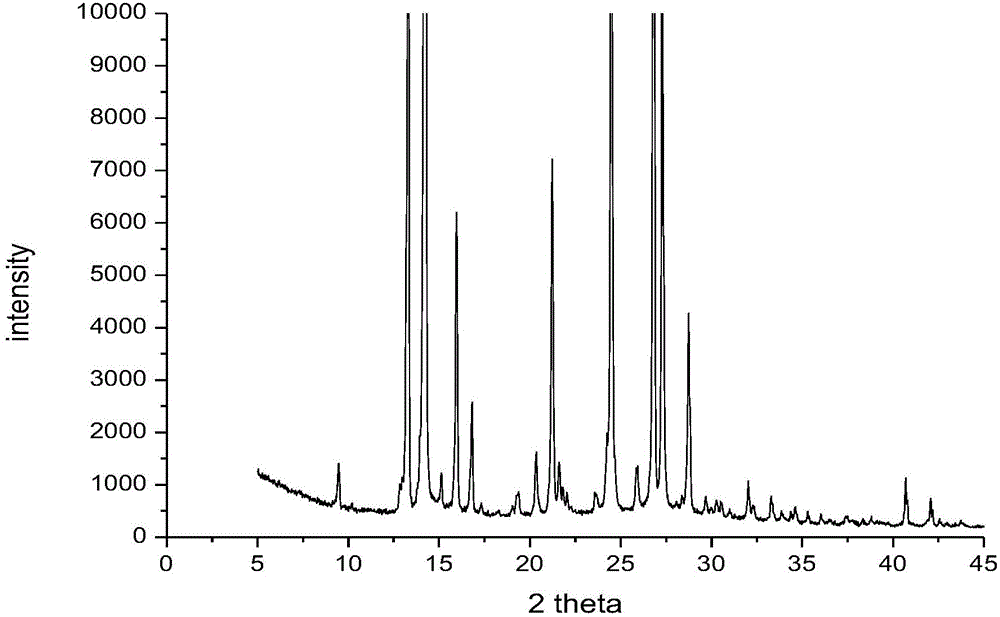
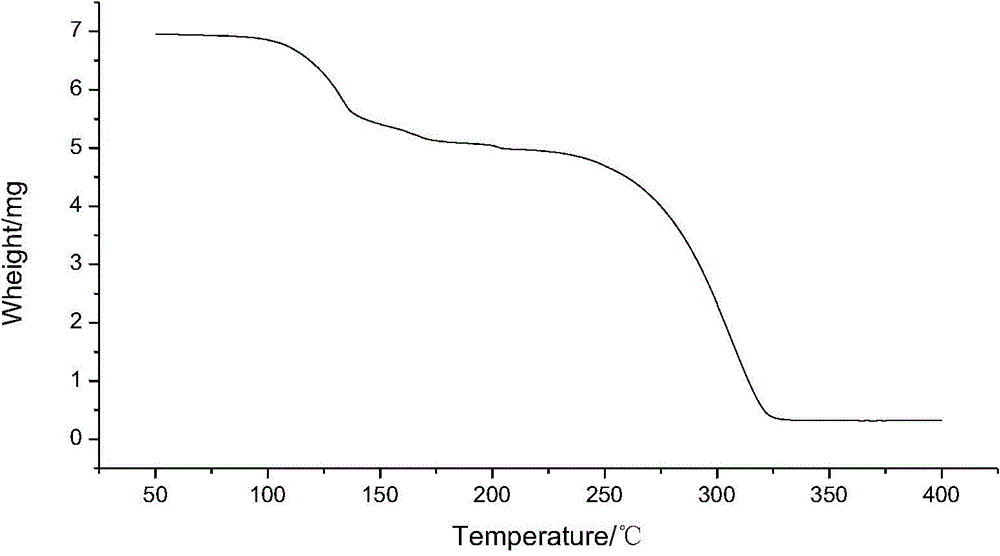
Dicyclanil medicine crystal form II and preparing method thereof
A technology of desiginil and drugs, applied in the field of medicinal chemistry, can solve the problems of insufficient stability of crystal form A, long reaction time and high temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation steps of the crystal form II of decinil drug according to the present invention are as follows:
[0045] (1) Dissolve dexinil in N,N-dimethylformamide solvent, heat and dissolve until it reaches a supersaturated state, stir for 30-60 minutes, and filter;
[0046] (2) Use a thin film to seal the mouth of the container containing the decinil solution, set volatilization holes in the thin film, let it stand for 5-7 days for volatilization, and colorless flaky crystals begin to precipitate in the container, and the crystals are obtained by separating them.
[0047] The present invention further provides a specific preparation method of crystal form II of decinil drug: dissolve 1.3-1.7 mmol of decinil in N,N-dimethylformamide solvent, heat and stir on a stirrer for 30 minutes ~60min, filter; seal the mouth of the beaker with a film of plastic wrap, poke a few small holes in the film with a needle, and let it stand for 5-7 days to evaporate, and colorless flak...
Embodiment 1
[0048] Example 1: Synthesis of crystalline form II using decinil and N,N-dimethylformamide:
[0049] Weighing:
[0050] Add 300 mg of crystalline form A dexinil, and weigh it accurately with an analytical balance.
[0051] Drug substance dissolution:
[0052] Use a pipette to accurately measure 20ml of N,N-dimethylformamide into a 50ml beaker, and stir for 30min.
[0053] Saturated solution cooling crystallization method:
[0054] At 153°C, when there is still a small amount that cannot be dissolved, take out the stirring bar, filter it into a clean 50ml transparent glass vial with analytical filter paper, seal the bottle mouth with plastic wrap, prick a few small holes with a needle, and let it stand for volatilization . After about 7 days, flaky colorless transparent crystals were precipitated in the bottle.
[0055] Toxic test on pests: the selected pests are adults of cotton boll weevil
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com