Method for recycling manganese and copper resources from waste lithium ion batteries
A lithium-ion battery and waste technology, which is applied in the field of recycling manganese and copper resources, can solve the problems of easy generation of waste gas, waste residue, high tank pressure, and poor material performance, so as to reduce the tank voltage and DC energy consumption, reduce the tank voltage, The effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
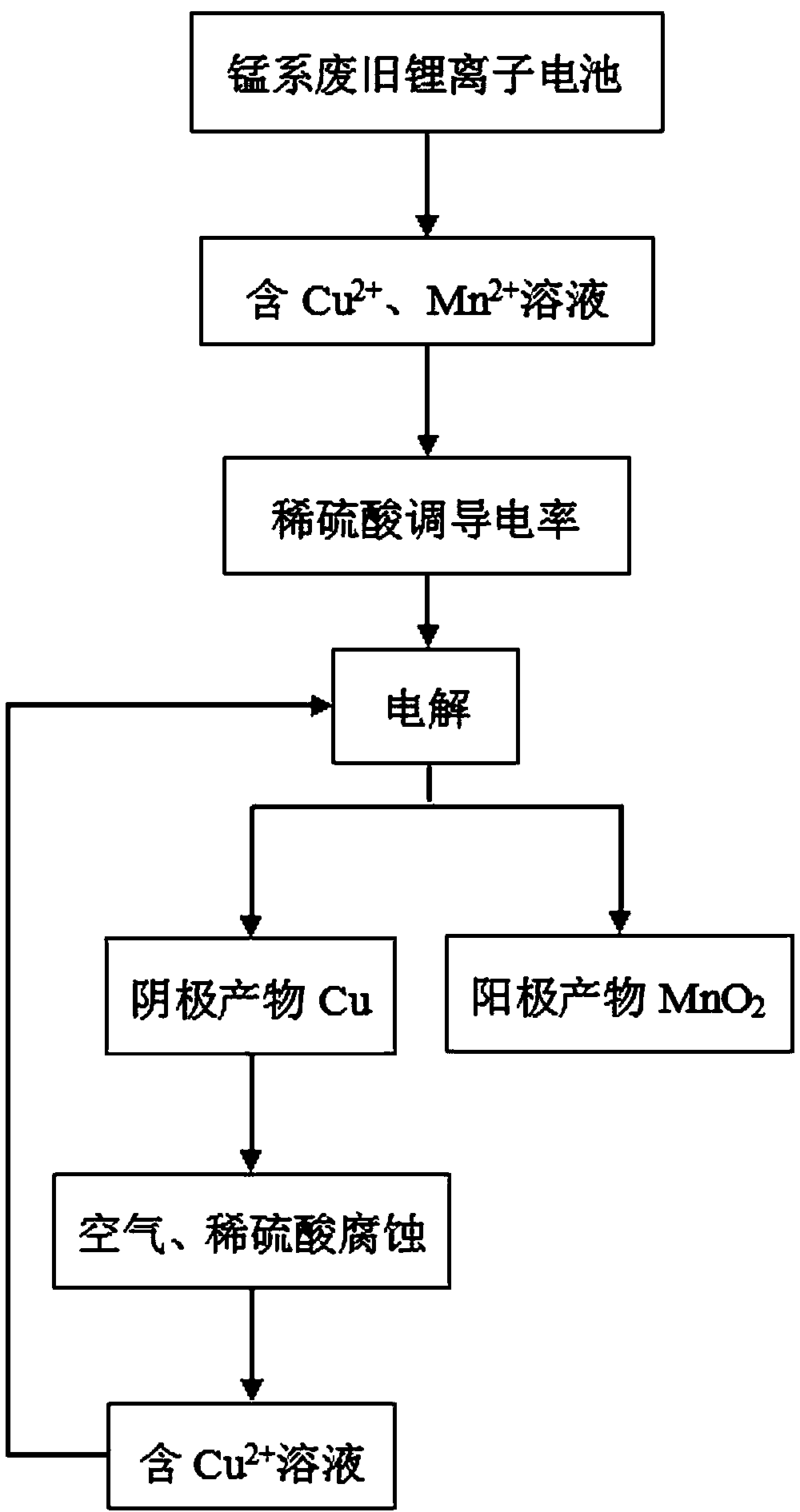
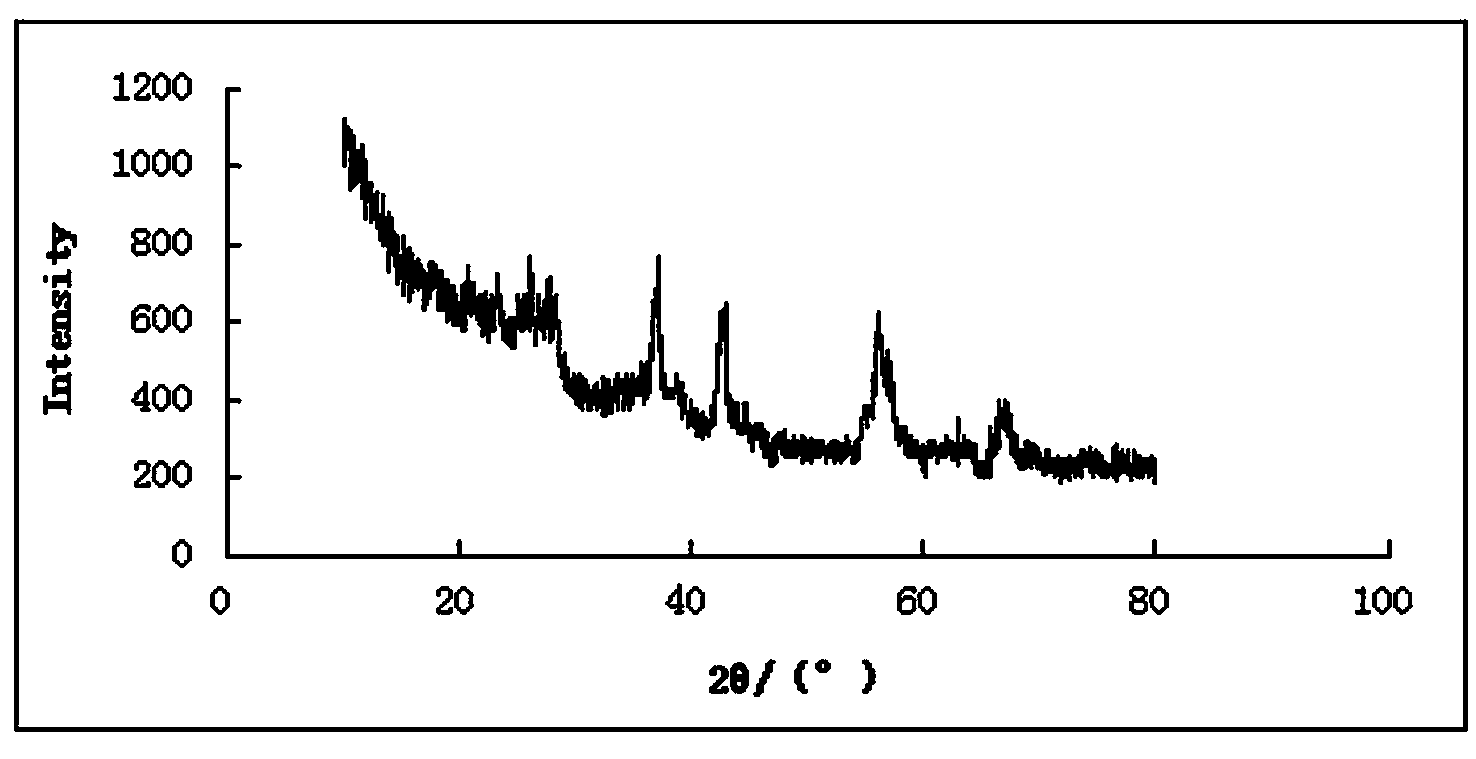
[0020] Take manganese-based waste lithium-ion batteries, first recover Co, Ni, Li and Al elements by acid dissolution method, and then separate to obtain Cu-containing 2+ , Mn 2+ A solution of two ions, adding an appropriate amount of dilute sulfuric acid to the solution to adjust the conductivity of the solution, the concentration of dilute sulfuric acid in the solution after adjustment is 0.1mol / L; the copper sheet is used as the cathode (effective electrode area 2 × 32.0cm 2 ), the graphite sheet is the anode (the effective electrode area is 2×32.2cm 2 ), with the above adjusted solution as the electrolyte, the adjusted current is 34.5mA (the anode current density is 0.5mA / cm 2 ) for electrolysis, the cell voltage range of the electrolysis process is 1.25~1.76V, after 1 hour of electrolysis, the product precipitated from the anode is analyzed by XRD, after analysis, the anode product is MnO 2 , the anode product MnO 2 As a product recovery, the Cu precipitated at the cat...
Embodiment 2
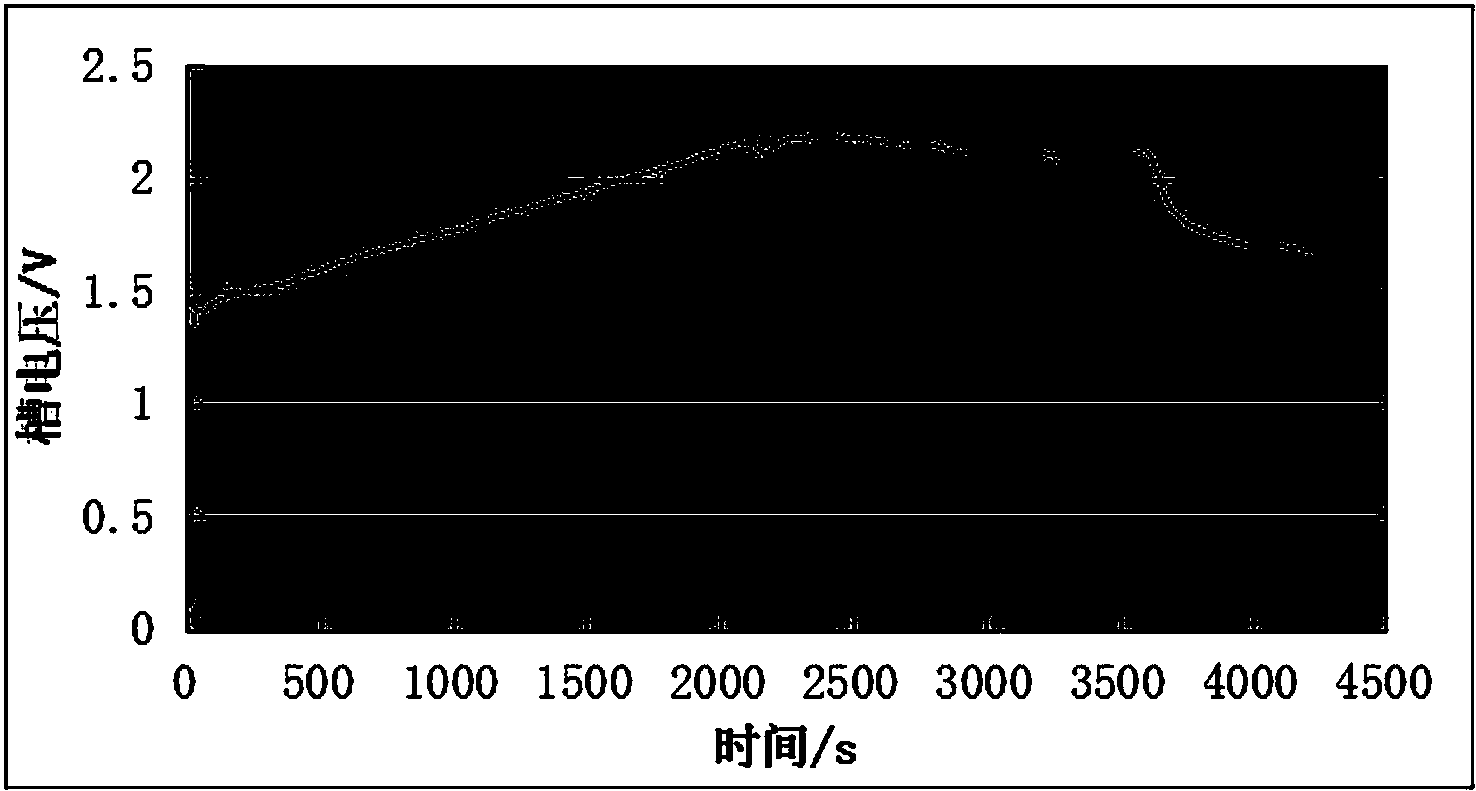
[0022] Take manganese-based waste lithium-ion batteries, first recover Co, Ni, Li and Al elements by acid dissolution method, and then separate to obtain Cu-containing 2+ , Mn 2+ A solution of two ions, adding an appropriate amount of dilute sulfuric acid to the solution to adjust the conductivity of the solution, the concentration of dilute sulfuric acid in the solution after adjustment is 0.5mol / L; the copper sheet is used as the cathode (the effective electrode area is 2×32.0cm 2 ), the graphite sheet is the anode (the effective electrode area is 2×32.2cm 2 ), with the above adjusted solution as the electrolyte, the adjusted current is 400mA (the anode current density is 5.8mA / cm 2 ) for electrolysis, the change of cell voltage during electrolysis is as follows figure 2 As shown, the voltage of the electrolytic cell is 1.28~2.20V; after electrolysis for 1 hour, 0.749g of the product is precipitated at the anode, and 0.453g of copper is precipitated at the cathode; the pr...
Embodiment 3
[0024] Take manganese-based waste lithium-ion batteries, first recover Co, Ni, Li and Al elements by acid dissolution method, and then separate to obtain Cu-containing 2+ , Mn 2+ A solution of two kinds of ions, adding an appropriate amount of dilute sulfuric acid to the solution to adjust the conductivity of the solution, the concentration of dilute sulfuric acid in the solution after adjustment is 5.0mol / L; the copper sheet is used as the cathode (the effective electrode area is 2×32.0cm 2 ), the graphite sheet is the anode (the effective electrode area is 2×32.2cm 2 ), with the above adjusted solution as the electrolyte, the adjusted current is 3450mA (the anode current density is 50mA / cm 2 ) for electrolysis, the range of cell voltage in the electrolysis process is 1.31~2.23V, after electrolysis for 1 hour, the product precipitated from the anode is analyzed by XRD, after analysis, the anode product is MnO 2 , the anode product MnO 2 As a product recovery, the Cu precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com