Insecticidal lipid agents isolated from entomopathogenic fungi and uses thereof
A technology of lipids and fungi, applied in the field of insect control, can solve the problems of less biological control agents and uneconomical
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0312] Example 1 - Biological Assay for Insect Control
[0313] This example describes the development of a robust test to determine the insecticidal efficacy of various lipids.
[0314] Criteria: 1) effectiveness, 2) susceptibility, and 3) ease of use were used to develop and evaluate tests for target insects.
[0315] All experiments used the target insect Myzus persicae (green peach aphid, Hemiptera) reared on cabbage plants in a normal room. A sample of the lipid fraction was used to inoculate aphids and transfer the aphids to a cabbage leaf on the surface of a 1% water agar plate using 0.05% Tween80 as a wetting agent between the leaf and the agar . Use aphids of mixed ages, usually between 30-50 petri dishes.
[0316] A hand-held Paasche spray gun was adapted to take microvolumes and was used to nebulize 300 μl of test or control solutions. Subsequently, plates with treated aphids were kept at 20°C, 12h light: 12h dark, and checked daily. Remove dead ones. Inoculat...
Embodiment 2
[0317] Example 2 - Biological Assays for Insect Control
[0318] This example describes the development of a large scale bioassay to determine insecticidal efficacy.
[0319] A test for multiple target insects was developed and evaluated using the criteria: 1) effectiveness, 2) susceptibility, and 3) ease of use. Test 4 insect systems: whitefly, cabbage, mealworm and mosquito.
[0320] 1. Whitefly larvae (Hemiptera)
[0321] Whiteflies are the main target species and there is often a lack of suitable control agents. Whitefly larvae were obtained from Bioforce Ltd (Auckland, New Zealand). Groups of approximately 100 larvae were inoculated by spray tower with samples of the K4B3 lipid fraction and control broth.
[0322] 2. Mealworm (Tenebrio molitor) larvae (Coleoptera)
[0323] Tenebrio molitor was obtained from a biological supplier. Ten larvae were sprayed with K4B3 lipid samples or water controls and monitored for 2 weeks.
Embodiment 3
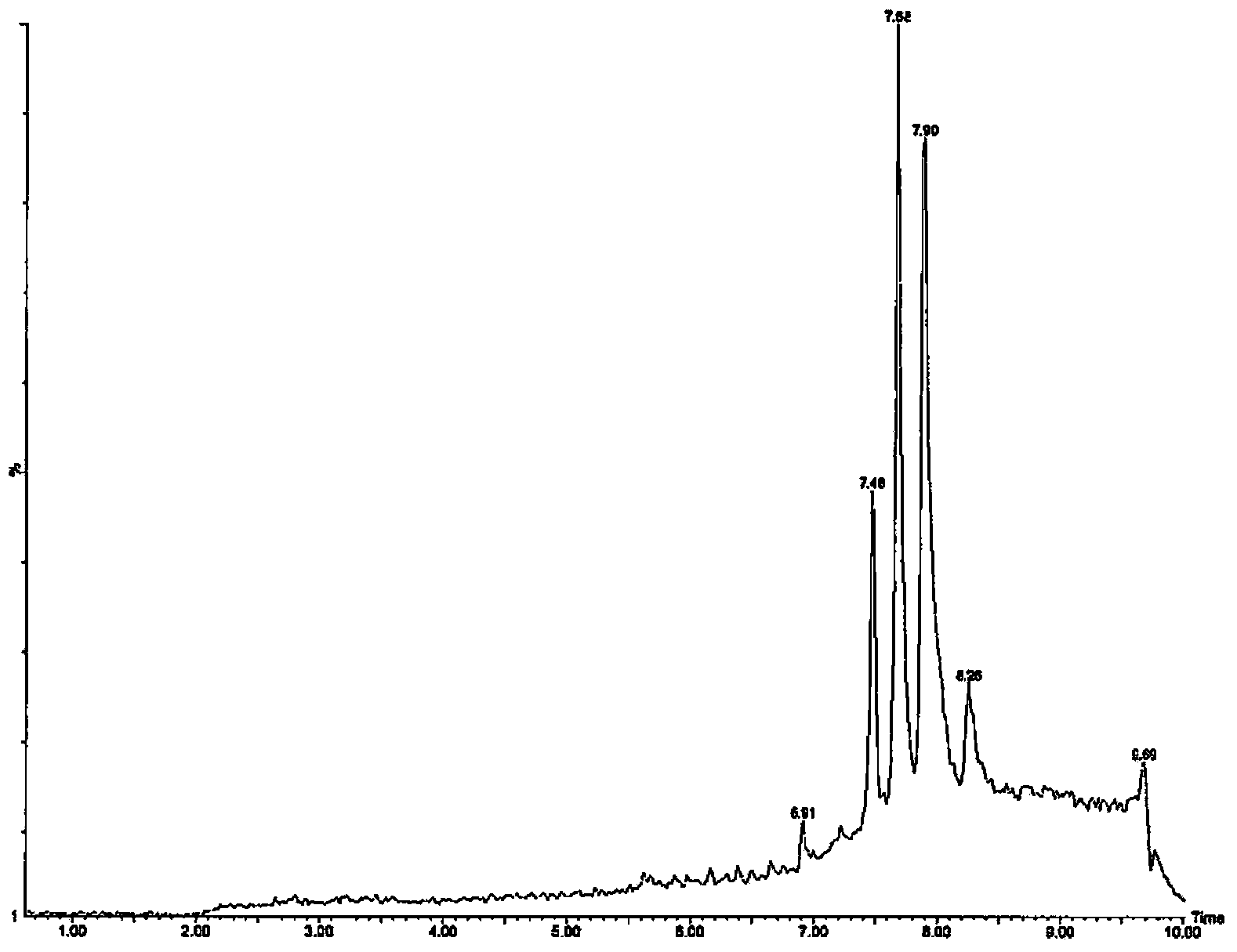
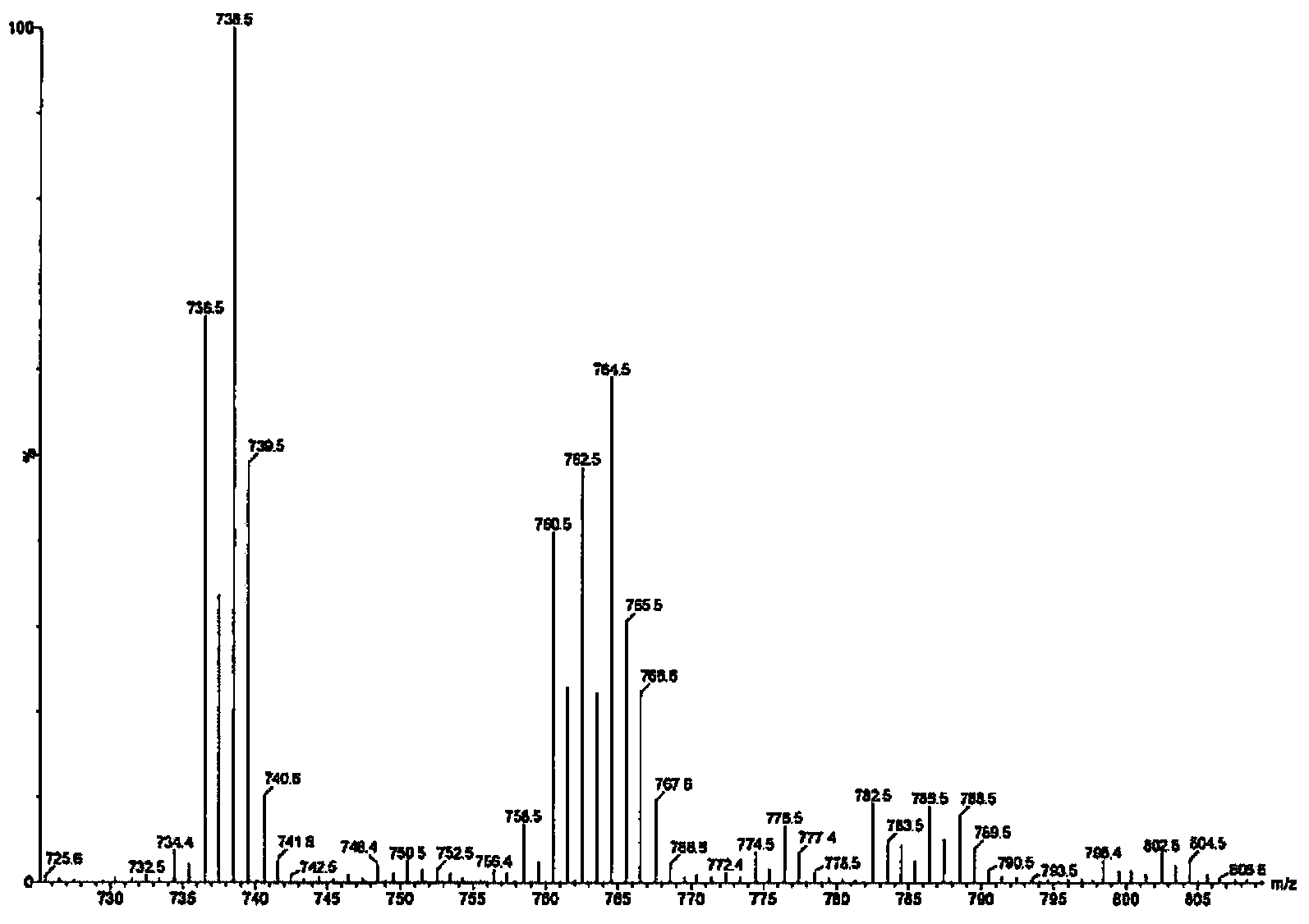
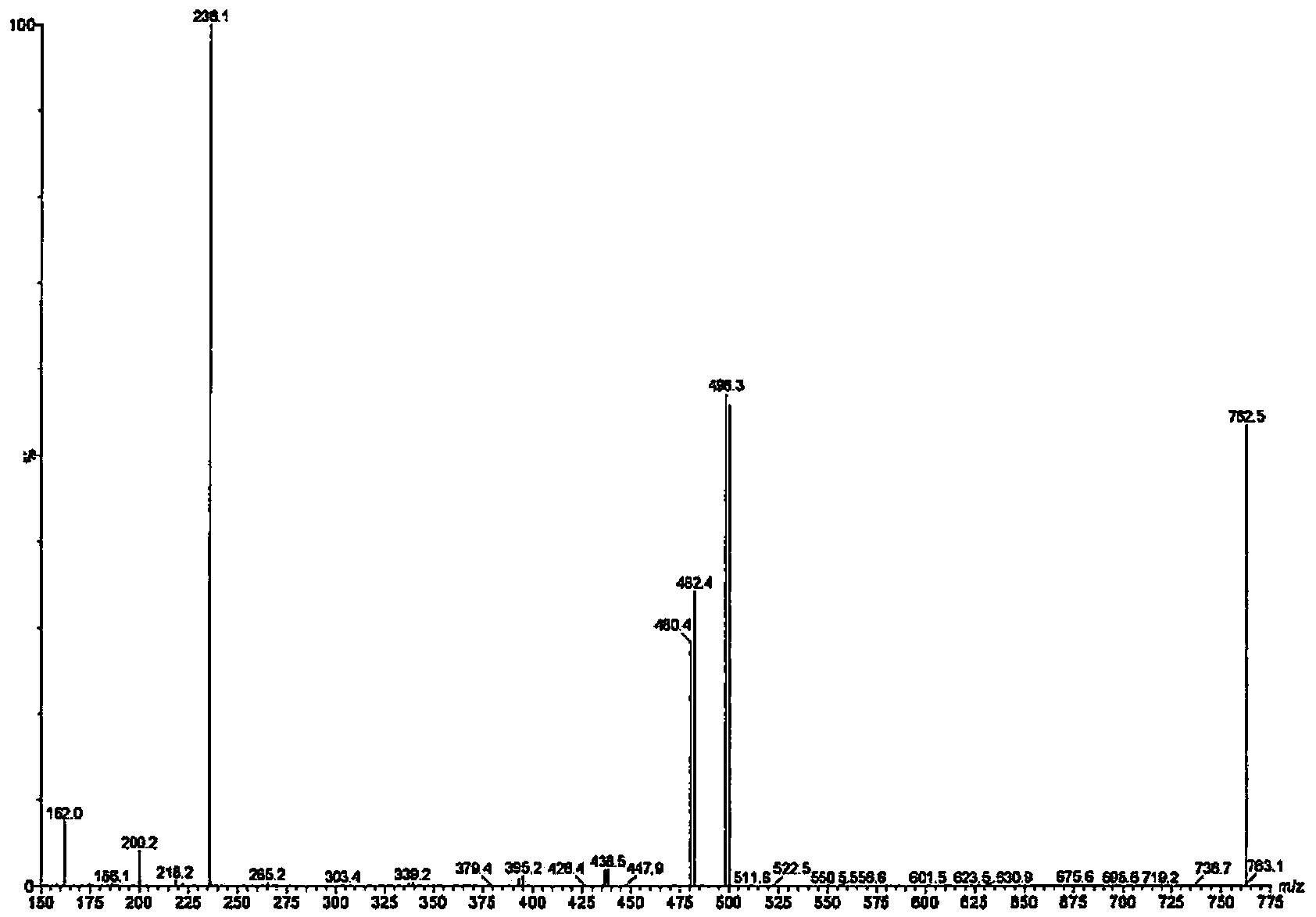
[0330] Example 3 - Identification of Pesticide Lipids
[0331] introduction
[0332] This example describes the identification of insecticide lipids prepared from Beauveria bassiana K4B3.
[0333] method
[0334] Cultures of Beauveria bassiana K4B3 were grown in shake flasks and 200L biofermentors. Freeze-dried samples were extracted with methanol and dried. The residue was dissolved in 50% ethanol and extracted with dichloromethane. Wash the dichloromethane with water to remove unwanted co-extractants. A small portion of the remaining dichloromethane extract was infected, redissolved in methanol, and subjected to MS analysis.
[0335] Fractional distillation was performed on a Strata SI-1 Silica column (Phenomenex). Crude samples were dissolved in a small amount of dichloromethane, loaded onto a Silica column, and used a mixture of isopropanol and dichloromethane (100% dichloromethane to 100% isopropanol in multiple steps) Elution was followed by a mixture of methanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com