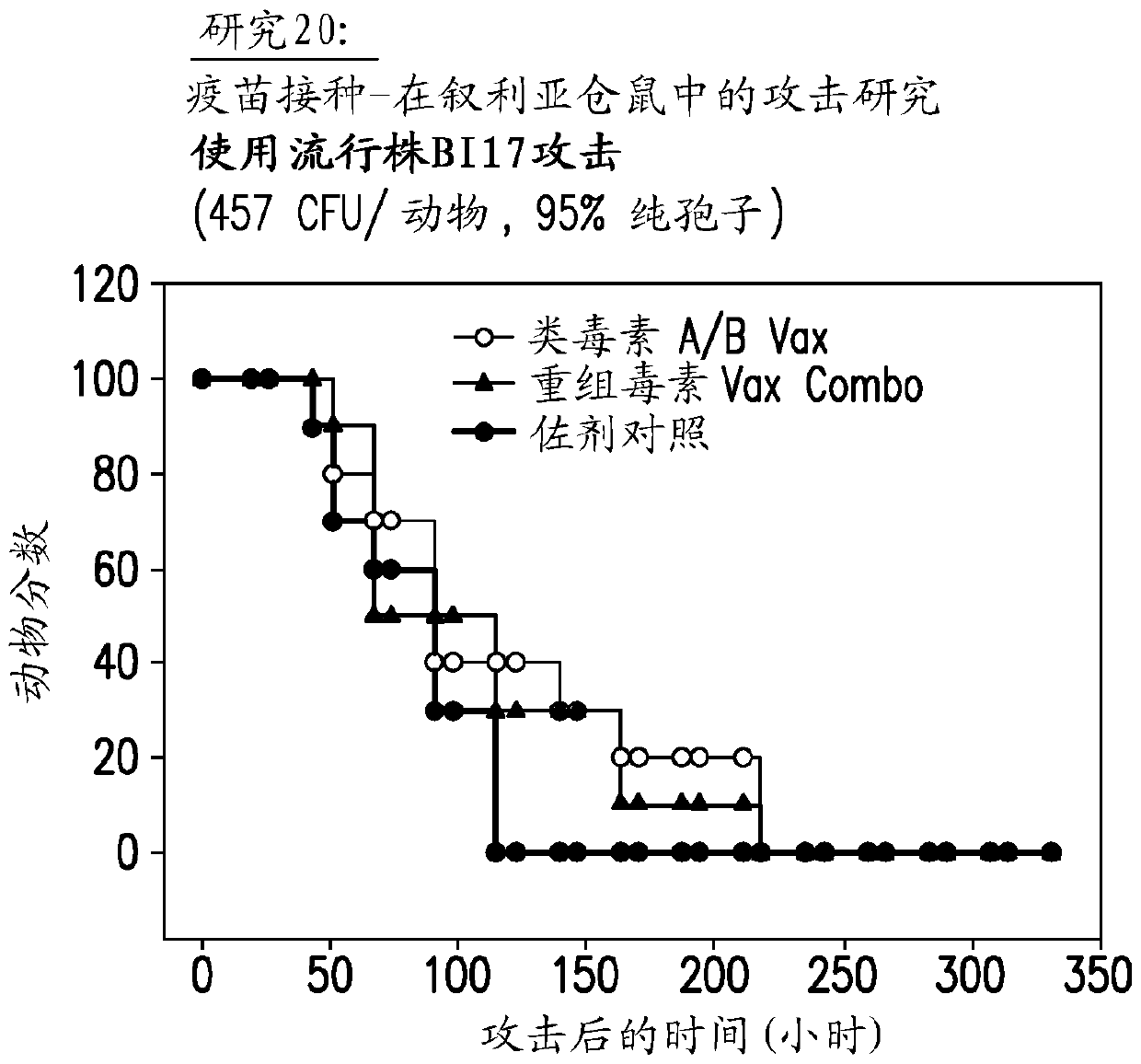
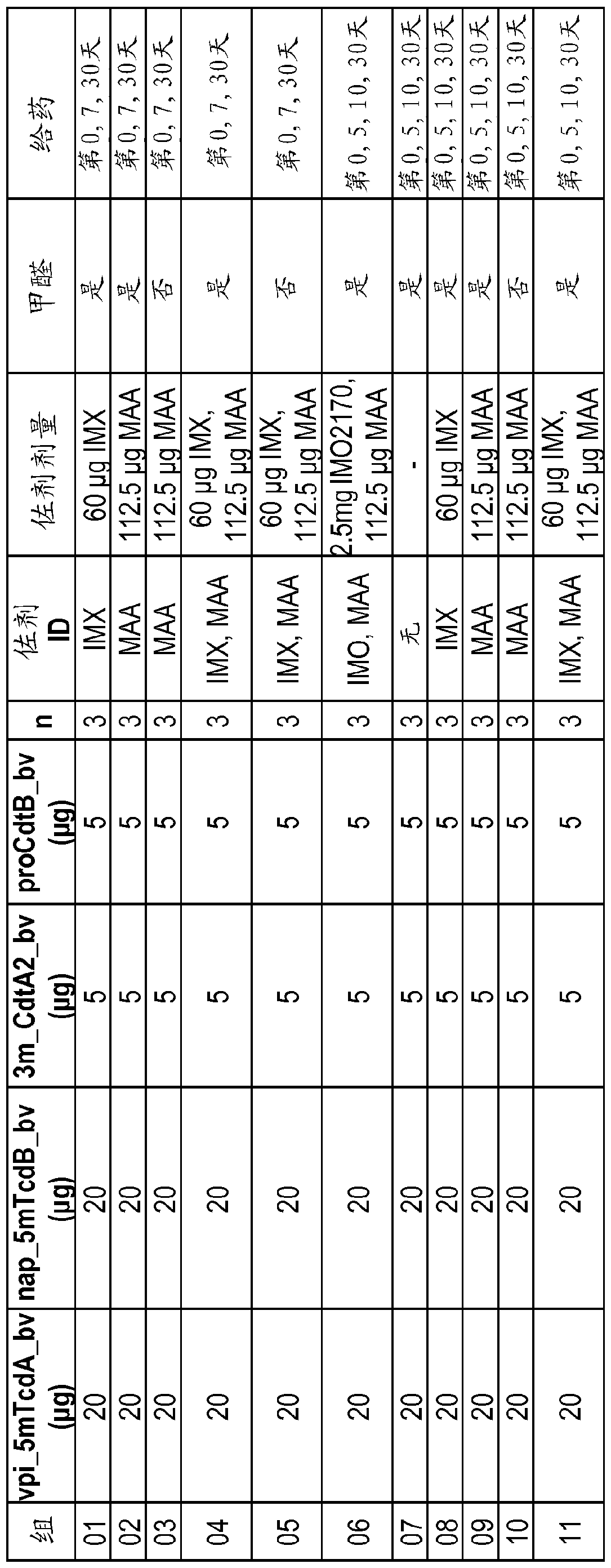
Vaccine against Clostridium difficile containing recombinant toxin
A technology of binary toxin and composition, applied in the field of Clostridium difficile vaccine, can solve problems such as failure to eliminate CDAD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Expression of Clostridium difficile TcdA and TcdB mutants in E. coli.
[0187] The C. difficile native A and toxin B encoding nucleotide sequences derived from strain VPI10463 were subjected to mutagenesis using the Quick Change® XL Site-Directed Mutagenesis Kit (Stratagene Corp., La Jolla, CA). As shown in Figure 3, several mutant toxins with different combinations of mutations were prepared. Mutants were expressed and purified from E. coli. In addition, the codon-optimized nucleotide sequence for the E. coli expression system was obtained from GenScript (GenScript USA, Inc., Piscataway, NJ). Of the mutants shown in Figure 3, only the E. coli codon-optimized triple mutant TcdB (W102A, D288A, E515Q) was expressed in full-length form. Additional constructs consisting only of the catalytic domain expressed in E. coli were tested.
[0188] Construct expression in E. coli was assessed by SDS-PAGE. Such as Figure 4 As shown in , expression analysis of the Tcd mutant en...
Embodiment 2
[0190] TcdA and TcdB enzyme activity assays with RhoA glucosylation assay.
[0191] Toxicity of recombinant TcdA and TcdB enzyme domains was compared in vitro using the RhoA glucosylation activity assay. Briefly, the protein was mixed at two different concentration levels (1 and 10 µg / mL) with a reaction buffer containing substrate (recombinant RhoA-GTPase), cofactor (MnII) and radiolabeled co-substrate (UDP-14C-glucose). Reactions were incubated at 37°C for 90 minutes, and ice-cold 10% trifluoroacetic acid was used to precipitate the protein along with any incorporated 14C. Precipitated material was recovered by filtration and the filtrate was quantified in a liquid scintillation counter.
[0192] The measurement results about TcdB are shown in Figure 5 middle. The triple mutant W102A, D288A, E515Q catalytic domain appeared to retain about 10% of the glucosylation activity of the native catalytic domain, an activity comparable to that of the W102 deletion mutant. No det...
Embodiment 3
[0195] Expression of TcdA and TcdB in baculovirus expression system.
[0196] To overcome the difficulties encountered in the expression of recombinant TcdA and TcdB, several expression platforms were evaluated. E. coli was initially tested; however, the inventors were unable to express 3mTcdA using this system, and although 3mTcdB was successfully expressed, it was partially degraded. The present inventors also evaluated the expression of 4mTcdA / B in E. coli, and again, 4mTcdA could not be successfully produced in this host system, but 4mTcdB was produced at high levels (~1.6 g / L). The Bacillus megaterium expression system was also evaluated and successfully produced low to moderate amounts of 5mTcdA (~75mg / L) and 5mTcdB (~200-700mg / L).
[0197] Based on previous examples of expressing full-length TcdA and TcdB in this organism (Burger et al. Biochem Biophys Res.Commun . 307(3): 584-8(2003); Yang et al., BMC Microbiol. 8:192(2008)), evaluating Bacillus megaterium as an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com