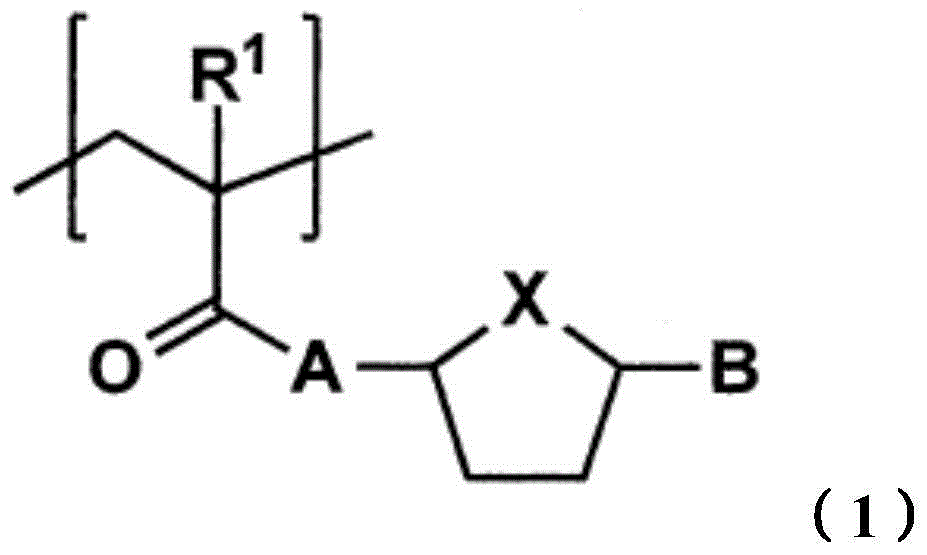
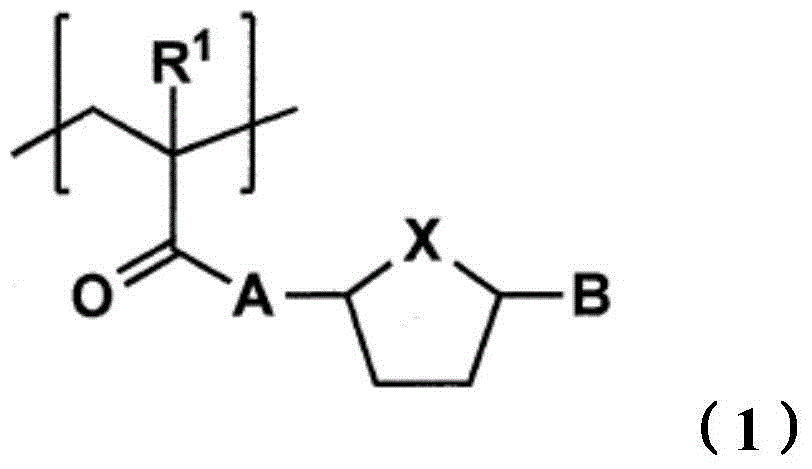
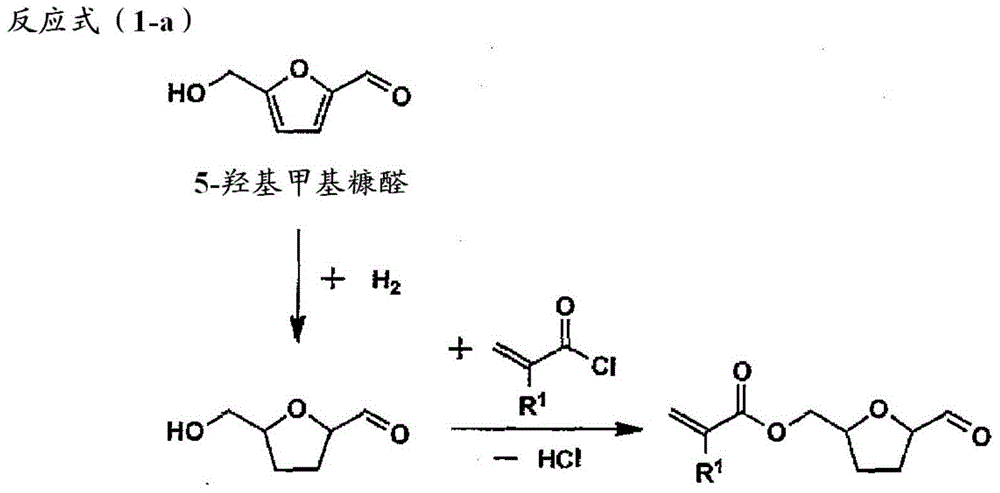
Toner for electrostatic image development, production method of the toner and image formation method
A technology of electrostatic charge image and manufacturing method, which is applied in the directions of developer, electrography, instrument, etc., can solve the problems of charge quantity fluctuation, browning, color development obstacle of color toner, etc. The effect of the change in electricity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0200] Hereinafter, the present invention will be specifically described with reference to examples, but the present invention is not limited thereto. It should be noted that the expressions of "part" or "%" are used in the examples, and unless otherwise specified, "parts by mass" or "% by mass" are used.
[0201] [Synthesis of Monomer]
[0202]
[0203] To a solution (200ml) of 5-hydroxymethylfurfural (12.6g, 100mmol), triethylamine (29.2ml, 210mmol) in dichloromethane, under nitrogen flow, add methacryloyl chloride (8.5ml) dropwise at 0°C , 105 mmol), stirred at room temperature for one day to prepare a reaction mixture. Use 1N-HCL (200ml×2), saturated NaHCO 3 aqueous solution (200ml × 1), saturated NaCl aqueous solution (200ml × 1) to wash the reaction mixture, with anhydrous MgSO 4 Dry and filter. The solvent of the filtrate thus obtained was distilled off under reduced pressure to obtain a crude product of monomer A. It was separated by silica gel column chromatogr...
preparation example 1
[0223] (1) Preparation process of colorant particle dispersion
[0224] 11.5 parts by mass of sodium lauryl sulfate was added to 160 parts by mass of ion-exchanged water, dissolved and stirred to prepare an aqueous surfactant solution. To this aqueous surfactant solution, 15 parts by mass of a colorant (C.I. Pigment Orange 36) was gradually added, followed by dispersion treatment using a mechanical disperser "CREARMIX" (manufactured by M-TECHNIQUE Co., Ltd.) to prepare fine particles in which the colorant was dispersed. Colorant particle dispersion [Or].
[0225] (2) Preparation of resin particle dispersion [A1]
[0226] (a) Step 1 aggregation
[0227] In the reaction vessel equipped with a stirring device, a temperature sensor, a condenser, and a nitrogen introduction device, add a surface active compound obtained by dissolving 4 parts by mass of polyoxyethylene (2) sodium lauryl ether sulfate in 3000 parts by mass of ion-exchanged water. The solution was prepared, and the...
preparation example 2
[0240] An orange toner [B1] was prepared in the same manner as in Preparation Example 1 of the orange toner except that the monomer A was changed to the monomer B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com