High-immobilization-tendency heparinase I coding gene and protein thereof
A technology encoding gene and heparinase is applied in the field of easy immobilization of heparinase I encoding gene and its protein, and can solve the problems of low immobilization efficiency and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Deletion of the expression of the heparanase I protein HepA-1.
[0022] 1. Construction of the expression vector pMal-HepA-1-chBD.
[0023] The construction of the template PMal-HepA refers to the patent, the patent number is CN 1312183C.
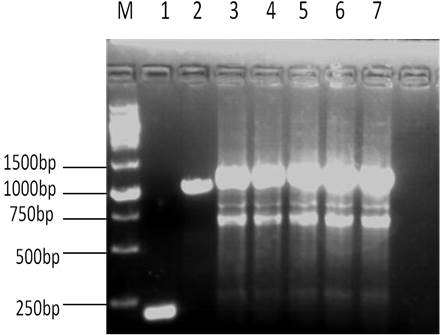
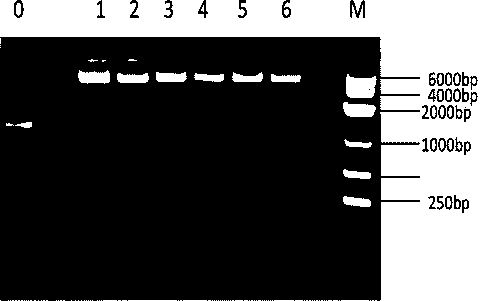
[0024] The specific process of constructing the expression vector pMal-HepA-1-chBD is as follows: the constructed PMAL-HepA and the chitin-binding domain plasmid PMD19T are used as templates, and the upstream and downstream primers used are GACTGGATCCcaggccgacagtgctaag (the underlined base is BamHI restriction site), and GGTCAGGCCAAGTTTtctggcagtttcgctgt 3 and, with 5 CAGCGAAACTGCCAGAAAACTTGGCCTGACCGGT and 5 GACTAAGCTTCTATTGAAGCTGCCACAAGGC 3, introduce BamHI and HindIII restriction sites respectively, and then recover and purify PMAL-HepA-1 and chitin-binding domain plasmid The PMD19T-chBD PCR product was used as a template to amplify the coding gene with GACTGGATCCcaggccgacagtgctaag and GACTAAGCTTCTATTGAAGCTGCCACAAGGC 3 ...
Embodiment 2
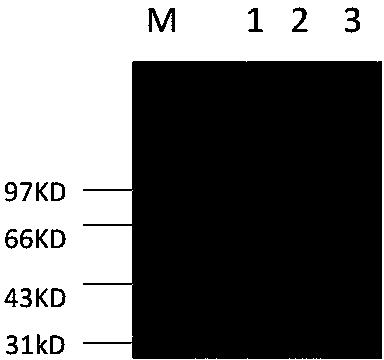
[0028] Example 2. Purification of heparanase I protein HepA-1-chBD by amylose column and determination of heparanase HepA-1-chBD activity.
[0029] 1. Purification of Heparanase Ⅰ protein HepA-1-chBD.
[0030] The fusion partner (fusion partner) maltose binding protein MBP used in the present invention can achieve one-step separation with amylose affinity adsorption. The specific affinity separation steps are as follows: after induction of 0.7mmol IPTG-induced bacterial expression for 20 hours, the bacterial solution was centrifuged at 8000rpm for 10min, and the filtrate was discarded and resuspended in 40ml 20mmol Tris. Ultrasonic 5s, intermittent time 5s, ultrasonic 200 cycles), centrifuged at 18000rpm.30min. After centrifugation, the supernatant was passed through a 20ml pre-equilibrated amylose affinity separation column at 0.5ml / min, eluted and collected by a gradient of 10mM 0.5ml / min maltose.
[0031] The target protein can be eluted with 10 mM maltose at 10 column vo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com