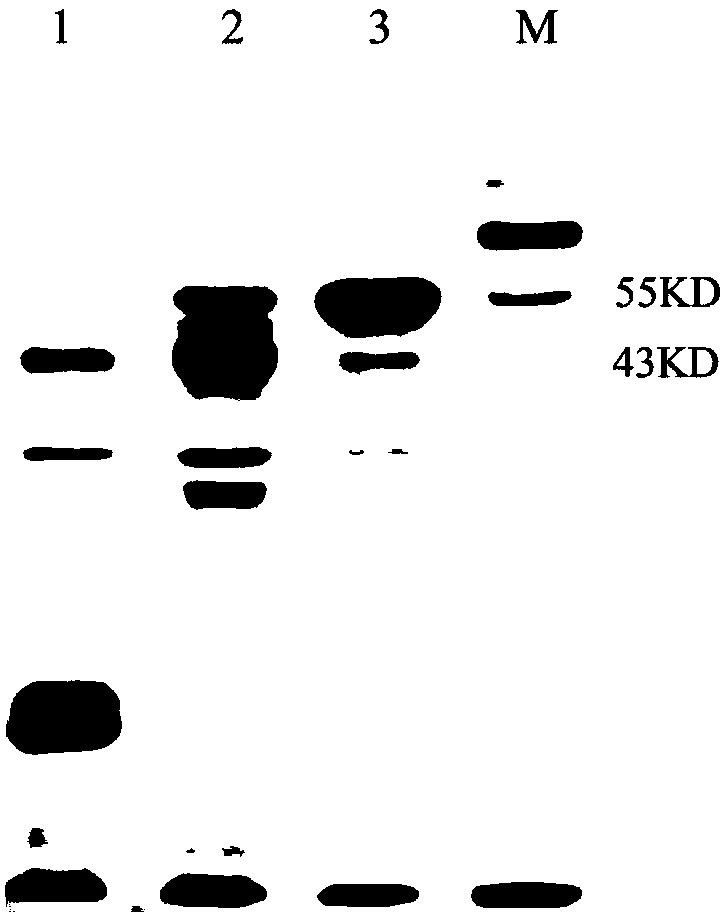High-expression and high-activity bacteroides thetaiotaomicron heparinase I fusion protein, and encoding gene and application thereof
A technology of Bacteroides polymorpha and fusion protein, applied in the field of genetic engineering, can solve the problems of low activity and low yield of heparanase I
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The present invention will be described in further detail below in conjunction with specific examples. The following examples are only descriptive, not restrictive, and cannot limit the protection scope of the present invention.
[0032] The raw materials used in the present invention, unless otherwise specified, are conventional commercially available products; the methods used in the present invention, unless otherwise specified, are conventional methods in the art.
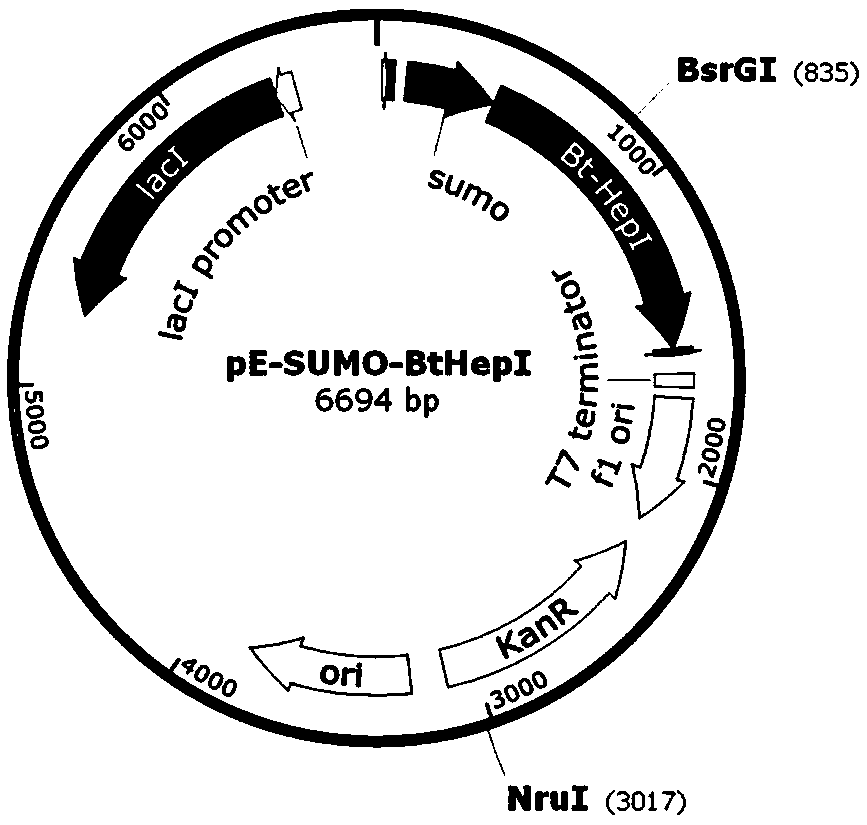
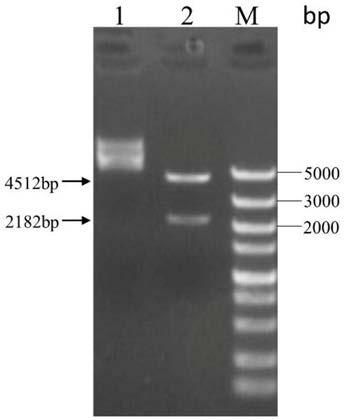
[0033] The bacterial strains and carriers used in the present invention can be: bacterial strain Escherichia coli DH5α, Escherichia coli Rosetta (DE3); pET-28a carrier and pE-SUMO carrier; The kit used in the present invention can be DNA cutting gel recovery kit , plasmid small extraction kit, Bradford protein concentration quantitative kit; the enzyme used in the present invention can be Fast PfuDNA polymerase, dNTPs, T4 ligase, restriction endonuclease BamHI, XhoI, NruI, BsrGI; PCR primer synthesis And s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com