Method for synthesizing forxiga intermediate
A synthesis method and intermediate technology, which are applied in the field of dapagliflozin synthesis, can solve the problems of high impurity content, low total yield and low product purity, and achieve the effects of reducing impurity content and improving purity and yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
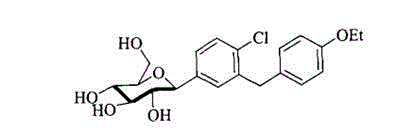
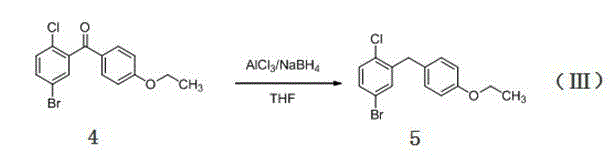
[0030] This embodiment relates to compound (5), namely 5-bromo-2-chloro-4 ′ -A kind of synthetic method of ethoxy diphenylmethane, comprises the steps:
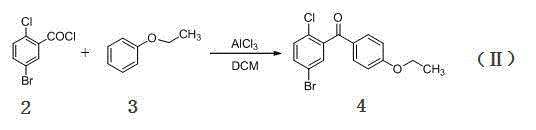
[0031] Step 1, add 5-bromo-2-chlorobenzoic acid (23.5 g, 0.1 mol) and 120 mL of dry dichloromethane in sequence in the flask, stir at room temperature, add 12.8 mL of oxalyl chloride (0.15 mol) in 3 times within 8 hours ), continue to stir at room temperature for 2 hours until the reaction solution is clarified and there is no more gas to be released, then evaporate the solvent and excess oxalyl chloride under reduced pressure to obtain the concentrated solution of 5-bromo-2-chlorobenzoyl chloride, that is, the compound ( 2), MS-EI(m / z): 253[M + ];
[0032] Step 2, dissolve 26 g of the above red transparent liquid (containing about 0.1 mol of 5-bromo-2-chlorobenzoyl chloride) in 40 mL of dry dichloromethane, cool to -7 °C, add phenetole (15.8 mL, 0.125 mol), and then added anhydrous aluminum trichloride (14.7 g, 0.11 mol) ...
Embodiment 2
[0035] This example relates to a compound (5) that is 5-bromo-2-chloro-4 ′ -A kind of synthetic method of ethoxy diphenylmethane, comprises the steps:
[0036] Step 1: Add 5-bromo-2-chlorobenzoic acid (23.5 g, 0.1 mol) and 120 mL of dry dichloromethane in sequence in the flask, stir at room temperature, add 8.5 mL of oxalyl chloride (0. 1 mol), after the addition, continue to stir at room temperature for 2 hours until the reaction solution is clarified and there is no more gas emission, then evaporate the solvent and excess oxalyl chloride under reduced pressure to obtain the concentrated solution of 5-bromo-2-chlorobenzoyl chloride, Namely compound (2), MS-EI (m / z): 253 [M + ];
[0037] Step 2, dissolve 26 g of the above red transparent liquid (containing about 0.1 mol of 5-bromo-2-chlorobenzoyl chloride) in 40 mL of dry dichloromethane, cool to -7 °C, add phenetole (18.9 mL, 0.15 mol), and then added anhydrous aluminum trichloride (14.7 g, 0.11 mol) in three batches, duri...
Embodiment 3
[0040] This example relates to a compound (5) that is 5-bromo-2-chloro-4 ′ -A kind of synthetic method of ethoxy diphenylmethane, comprises the steps:
[0041] Step 1: Add 5-bromo-2-chlorobenzoic acid (23.5 g, 0.1 mol) and 120 mL of dry dichloromethane in sequence in the flask, stir at room temperature, add 17 mL of oxalyl chloride (0. 2 mol l), after the addition, continue to stir at room temperature for 2 hours until the reaction solution is clear and no gas is released, then evaporate the solvent and excess oxalyl chloride under reduced pressure to obtain the concentrated solution of 5-bromo-2-chlorobenzoyl chloride , namely compound (2), MS-EI (m / z): 253 [M + ];
[0042] Step 2, dissolve 26 g of the above red transparent liquid (containing about 0.1 mol of 5-bromo-2-chlorobenzoyl chloride) in 40 mL of dry dichloromethane, cool to -7 °C, add phenetole (12.6 mL, 0.1 mol), and then added anhydrous aluminum trichloride (14.7 g, 0.11 mol) in three batches, during which the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com