Acryl multi-arm silane coupling agent and preparation method thereof
A silane coupling agent and aryl technology, which is applied in the field of aryl multi-arm silane coupling agent and its preparation, can solve the problems of weak binding force and few cross-linking points of the coupling agent, and achieve enhanced affinity. , Improve the bonding force, the effect of strong adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
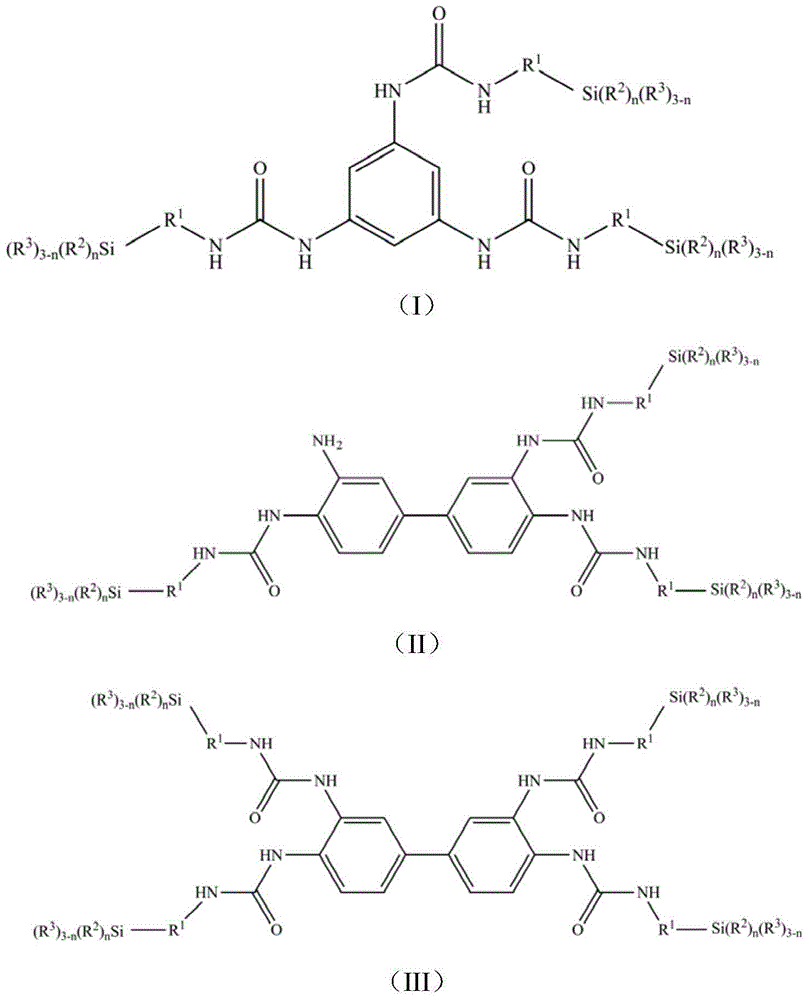
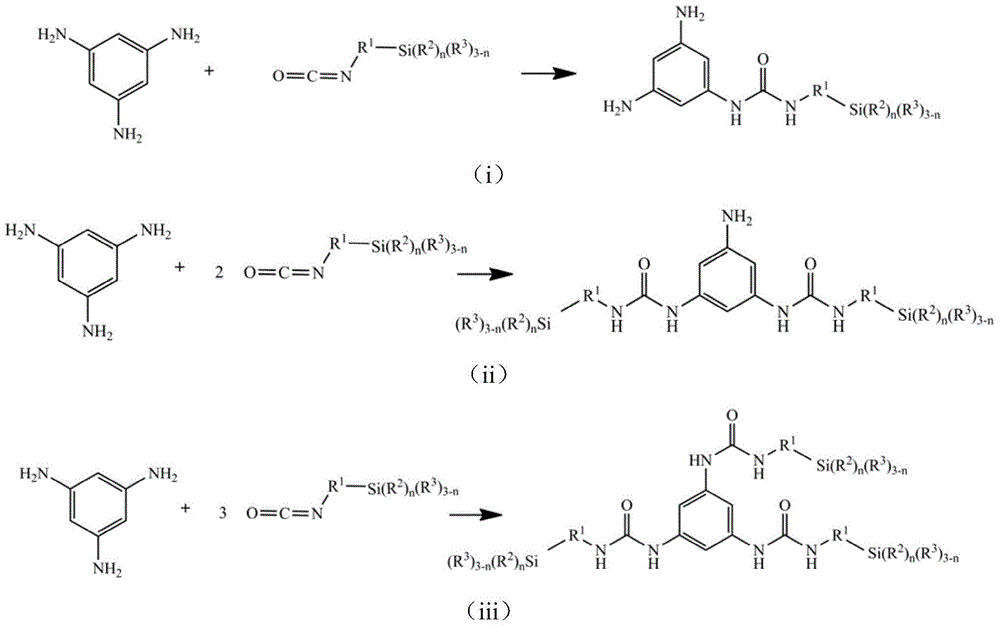
[0066] Add 0.1mol (12.32g) of 1,3,5-triaminobenzene and 200ml of dichloromethane into a four-neck round-bottomed flask equipped with a dropping funnel, a stirrer and a thermometer, blow nitrogen, stir, and add dropwise 0.3mol (61.58g) of isocyanate propyltrimethoxysilane, keep the reaction temperature at 10°C for 2h, then raise the temperature to 35°C for 2h, distill off dichloromethane to get the target compound - (I-1), yield was 98.7%.
[0067] For target compound-(I-1), by 1 H-NMR and 13 Confirmed by C-NMR analysis. 1 H-NMR (CDCl 3 ,ppm):δ7.87(s,C 6 h 3 ); δ6.0(s, NH), δ3.38(t, CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ),δ1.6(m,CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ),δ0.58(t,CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ),δ3.55(s,CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ); 13 C-NMR (CDCl 3 ,ppm): δ108.9(C 6 h 3 ), δ136.3(C 6 h 3 (substitution)), δ154.3 (C=O), δ45.6 (CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ), δ24.8 (CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ), δ12.5(CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ), δ50.2(CH 2...
Embodiment 2
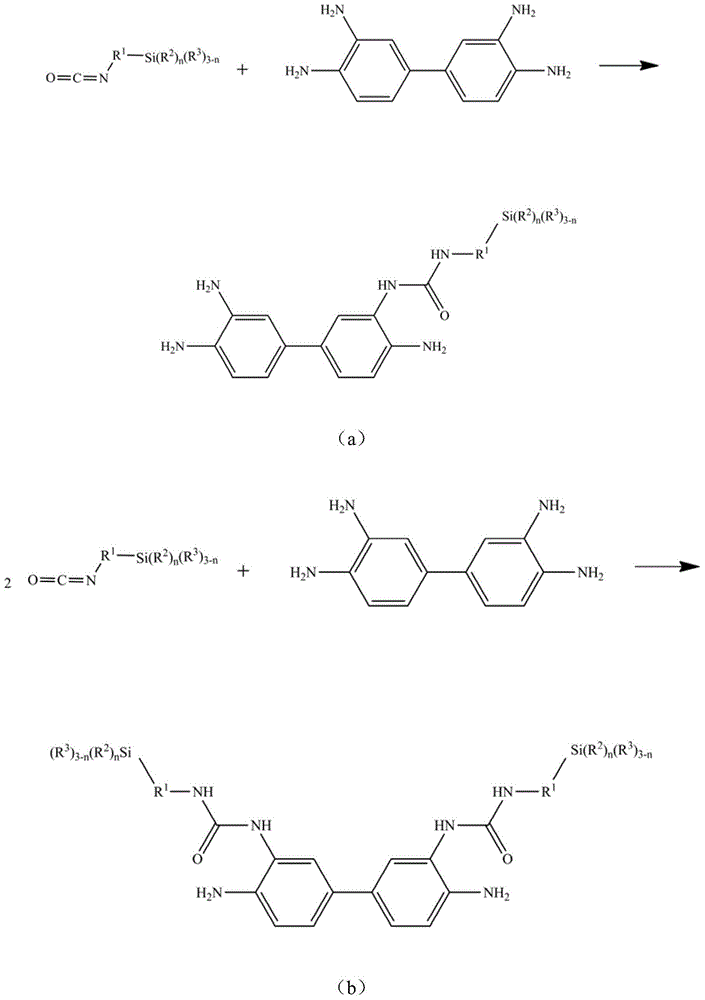
[0070] Add 0.1mol (21.43g) of 3,3',4,4'-biphenyltetramine and 200ml of dichloromethane into a four-neck round-bottomed flask equipped with a dropping funnel, a stirrer and a thermometer, blow nitrogen, and stir , add 0.3mol (61.58g) isocyanate propyltrimethoxysilane dropwise, keep the reaction temperature at 10°C for 2h, then raise the temperature to 40°C and continue the reaction for 3h, distill off the solvent dichloromethane to obtain the target compound -(II- 1), the yield is 95.6%.
[0071] For target compound-(II-1), by 1 H-NMR and 13 Confirmed by C-NMR analysis. 1 H-NMR (CDCl 3 ,ppm):δ6.87-8.05(m,C 12 h 6 ), δ6.08(s, NH), δ6.27(s, NH 2 ),δ3.42(t,CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ),δ1.58(m,CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ),δ0.61(t,CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ), δ3.58(s, CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ); 13 C-NMR (CDCl 3 ,ppm): δ114.8-142.3(C 12 h6 ), δ155.6 (C=O), δ46.8 (CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ),δ25.9(CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ), δ11.4 (C...
Embodiment 3
[0074] Add 0.1mol (21.43g) of 3,3',4,4'-biphenyltetramine and 300ml of dichloromethane into a four-necked round-bottomed flask equipped with a dropping funnel, a stirrer and a thermometer, blow nitrogen, and stir , add 0.4mol (82.11g) isocyanate propyltrimethoxysilane dropwise, keep the reaction temperature at 10°C for 2h, then raise the temperature to 45°C to continue the reaction for 3h, distill off the solvent dichloromethane to obtain the target compound -(III- 1), the yield is 96.7%.
[0075] For target compound-(III-1), by 1 H-NMR and 13 Confirmed by C-NMR analysis. 1 H-NMR (CDCl 3 ,ppm):δ7.51-8.05(m,C 12 h 6 ), δ6.05(s, NH), δ3.43(t, CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ),δ1.63(m,CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ),δ0.55(t,CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ),δ3.59(s,CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ); 13 C-NMR (CDCl 3 ,ppm): δ116.5-135.2(C 12 h 6 ), δ153.2 (C=O), δ46.2 (CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ), δ24.1(CH 2 CH 2 CH 2 Si(OCH 3 ) 3 ), δ13.2 (CH 2 CH 2 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com