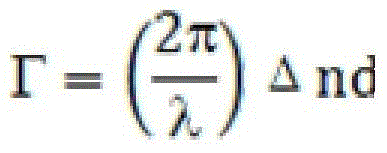A kind of high birefringence liquid crystal compound and composition thereof
A technology of liquid crystal composition and liquid crystal compound, applied in liquid crystal materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of high melting enthalpy, narrow temperature range of liquid crystal phase, crystallization, etc., and achieve high refractive index and low viscosity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
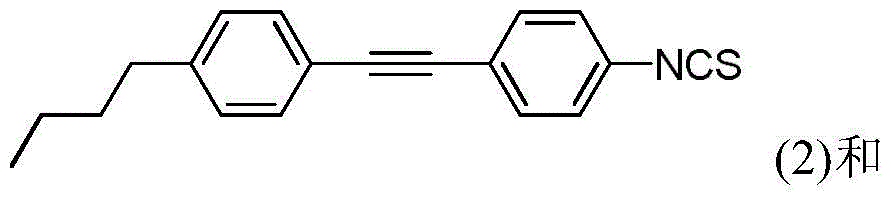
[0027] Preparation of 4-{[4-(3-butenyl)phenyl]ethynyl}-1-isothiocyanatobenzene:
[0028] The specific structural formula is as follows:
[0029]
[0030] The preparation process is as follows:
[0031] Step 1: Synthesis of 3-(4-bromophenyl)propanal
[0032]
[0033] Under the protection of nitrogen, add 51.4 grams (0.2mol) of p-bromophenylpropionaldehyde ethylene acetal, 102.8 grams of formic acid, and 150 ml of toluene in a 500ml three-necked flask, heat and reflux reaction for 1 hour to separate formic acid, and add 102.8 grams of neoformic acid, After repeated 3 times, the reaction was stopped and post-treatment was performed. The formic acid in the lower layer was separated, and the upper organic layer was washed with water until neutral, dried by adding anhydrous magnesium sulfate, filtered and concentrated to obtain 38 g of a yellow liquid with a GC purity of 99.46% and a yield of 89%.
[0034] Step 2: Synthesis of 1-bromo-4-(3-butenyl)benzene
[0035]
[00...
Embodiment 2
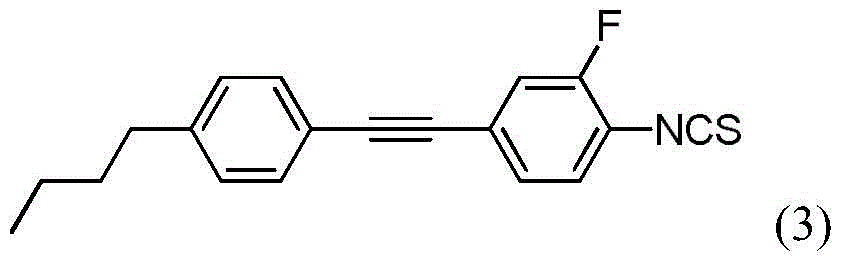
[0063] Using 2-fluoro-4-iodoaniline instead of 4-iodoaniline in Example 1, the same method was used to synthesize 4-{[4-(3-butenyl)phenyl]ethynyl}-2-fluoro-1 - Isothiocyanatobenzene.
[0064] Structure Identification:
[0065] 1 H NMR (δ, CDCl 3 ): 2.354~2.399(m, 2H), 2.730(t, 2H, J=6.5), 4.976~5.058(m, 2H), 5.795~5.876(m, 1H), 7.120~7.190(m, 3H), 7.236 ~7.296 (m, 2H), 7.424~7.441 (d, 2H, J=8.5).
[0066] IR(KBr)σ / cm -1 : 3076 (C=C, v), 3026 (Ar-H, v), 2976 (-CH 2 , v), 2852 (-CH 2 , v), 2208 (C≡C, v), 2044 (NCS, v), 1557, 1515 (Ar, v), 1422, 1209, 1104, 960, 870.
[0067] MS (70eV) m / z (%): 207.16(30), 265.95(100), 307.13(31).
[0068] The above structural identification data show that the synthesized compound is indeed 4-{[4-(3-butenyl)phenyl]ethynyl}-2-fluoro-1-isothiocyanate benzene. The liquid crystal phase transition characteristic temperature was measured by DSC at a heating rate of 5°C / min, and the result was: C46.54(N34.37)I, and the melting enthalpy was 55.1...
Embodiment 3
[0095] The liquid crystal composition containing the structure of Example 1 and the structure of Example 2 (see Table 5) includes the following components: wherein, "%" means "mass percentage", and the characteristics measured in the examples are as follows: Δn: at 25°C Birefringence anisotropy, V th : Threshold voltage measured in a 5 μm TN cell at 25°C, V sat : Saturation voltage measured in a 5 μm TN cell at 25 °C, T r : Rise time measured in a 5 μm TN cell at 25°C, T f : fall time measured in a 5 μm TN cell at 25 °C.
[0096] Table 5 embodiment 3 composition and performance
[0097]
[0098]
PUM
| Property | Measurement | Unit |
|---|---|---|
| isotropization temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| isotropization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com