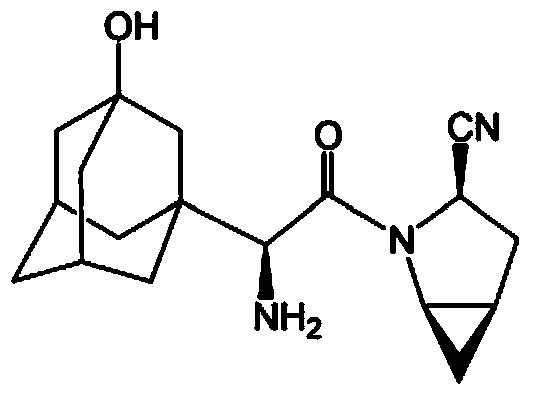
Preparation method for saxagliptin
A compound and dropping technology, applied in the preparation of sulfonamides, organic chemistry, etc., can solve the problems of difficult preparation and storage of biological enzymes, and achieve the effects of solving difficult preparation and storage, reducing production costs, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
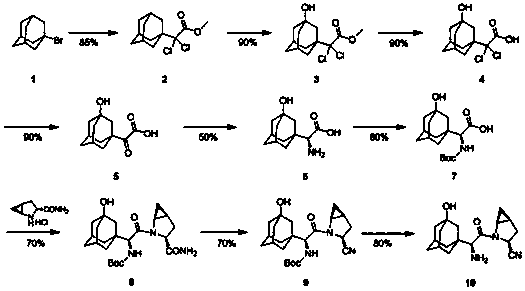
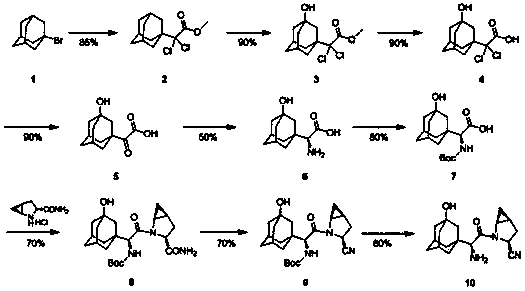
[0034] A preparation method of saxagliptin, compound 5 [2-(3-hydroxy-1-adamantyl)-2-1-oxoacetic acid (3)] to compound 7 (N-tert Butoxycarbonyl-3-hydroxyl-1-adamantylglycine) is carried out by replacing the reaction process of compound A to G in the following steps:
[0035](1) Put 130ml of absolute ethanol into a three-necked flask, cool it in an ice-salt bath to below -5°C, slowly add thionyl chloride (8ml, 110mmol) dropwise, and control the temperature not to exceed -5°C during the dropwise addition; After the addition was completed, compound A (20 g, 89 mmol) was added in batches, and the temperature was controlled to be lower than -5°C during the addition; after the addition was completed, the reaction solution was stirred at room temperature for 5 h, then the reaction solution was concentrated to 50 ml, and cooled to Below 0°C, add 100ml of toluene and 20g of triethylamine respectively; the reaction solution is stirred at 0°C for 30 minutes and then filtered, the filter c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com