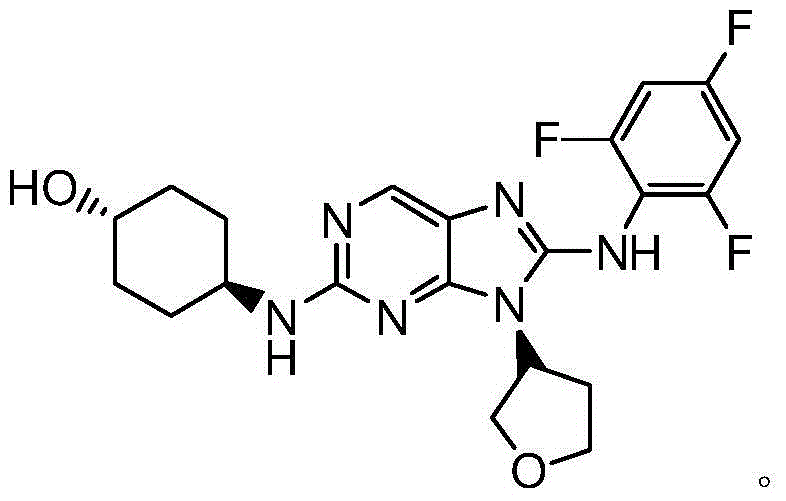
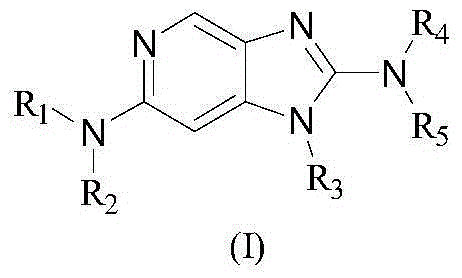
JNK (stress-activated kinases,SAPK) inhibitor compound
A compound, alkyl technology, applied in the field of JNK inhibitor compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196] Example 1 trans-4-(1-((S)-tetrahydrofuran-3-yl)-2-(2,4,6-trifluoroanilino)-1H-imidazo[4,5-c]pyridine Preparation of -6-ylamino)cyclohexanol (compound 1)
[0197]
[0198] 1. Preparation of (S)-2-chloro-5-nitro-N-(tetrahydrofuran-3-yl)pyridin-4-amine
[0199]
[0200] Dissolve 2,4-dichloro-5-nitropyridine (1.93g, 10.0mmol) and diisopropylethylamine (1.55g, 12.0mmol) in dichloromethane (30mL) in batches under ice-water bath Add (S)-3-aminotetrahydrofuran hydrochloride (1.35g, 10.9mmol), after the addition is complete, stir for half an hour, then transfer to room temperature for 24 hours of reaction, concentrate under reduced pressure, silica gel column chromatography (petroleum ether: ethyl acetate =5:1) 2.16g of yellow solid was obtained with a yield of 88.6%.
[0201] 2. Preparation of trans-4-(5-nitro-4-((S)-tetrahydrofuran-3-ylamino)pyridin-2-ylamino)cyclohexanol
[0202]
[0203] (S)-2-Chloro-5-nitro-N-(tetrahydrofuran-3-yl)pyridin-4-amine (2.16g, 8.86mmo...
Embodiment 2
[0218] Example 2 trans-N-methyl-4-(1-((S)-tetrahydrofuran-3-yl)-2-(2,4,6-trifluoroanilino)-1H-imidazo[4, Preparation of 5-c]pyridin-6-ylamino)cyclohexylcarboxamide (compound 3)
[0219]
[0220] 1. Preparation of trans-4-(tert-butoxycarbonylamino)cyclohexylcarboxylic acid
[0221]
[0222] Trans-4-amino-cyclohexylcarboxylic acid (3.1g, 21.6mmol), potassium carbonate (3.3g, 23.9mmol) and Boc2O (5.2g, 23.8mmol) in a mixed solution of acetone and water (100mL, v / v=5:1), reacted at room temperature for 18 hours, concentrated most of it, adjusted to acidity with citric acid, precipitated white solid, filtered with suction, washed with petroleum ether, and dried to obtain 4.86 g of white solid with a yield of 92.6%.
[0223] 2. Preparation of tert-butyl trans-4-(methylcarbamoyl)cyclohexylcarbamate
[0224]
[0225]Trans-4-(tert-butoxycarbonylamino)cyclohexylcarboxylic acid (4.86g, 20mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (13.4g, 69.9 mmol) and ...
Embodiment 3
[0239] Example 3 2-(trans-4-(1-((S)-tetrahydrofuran-3-yl)-2-(2,4,6-trifluoroanilino)-1H-imidazo[4,5- c] Preparation of pyridin-6-ylamino)cyclohexyl)propan-2-ol (compound 4)
[0240]
[0241] 1. Synthesis of trans-4-(dibenzylamino)cyclohexylcarboxylate benzyl ester
[0242]
[0243] Trans-4-aminocyclohexylcarboxylic acid (4.0g, 27.9mmol) and K 2 CO 3 (13.53g, 97.9mmol) was dissolved in 100mLTHF, then benzyl bromide (16.74g, 97.9mmol) was added dropwise, stirred at room temperature for 16h, the reaction solution was spin-dried, and passed through the column (PE / AE=1:4) to obtain light yellow Solid 6.2g, yield: 53.8%.
[0244] 2. Synthesis of 2-(trans-4-(dibenzylamino)cyclohexyl)propan-2-ol
[0245]
[0246] Dissolve trans-4-(dibenzylamino)benzylcyclohexylcarboxylate (4g, 9.67mmol) in dry 25mL THF, slowly add 32.3mL 3M methylmagnesium bromide ether solution (96.9 mmol), stirred at room temperature for 5h, slowly added 100mL saturated NH 4 Cl aqueous solution was ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com