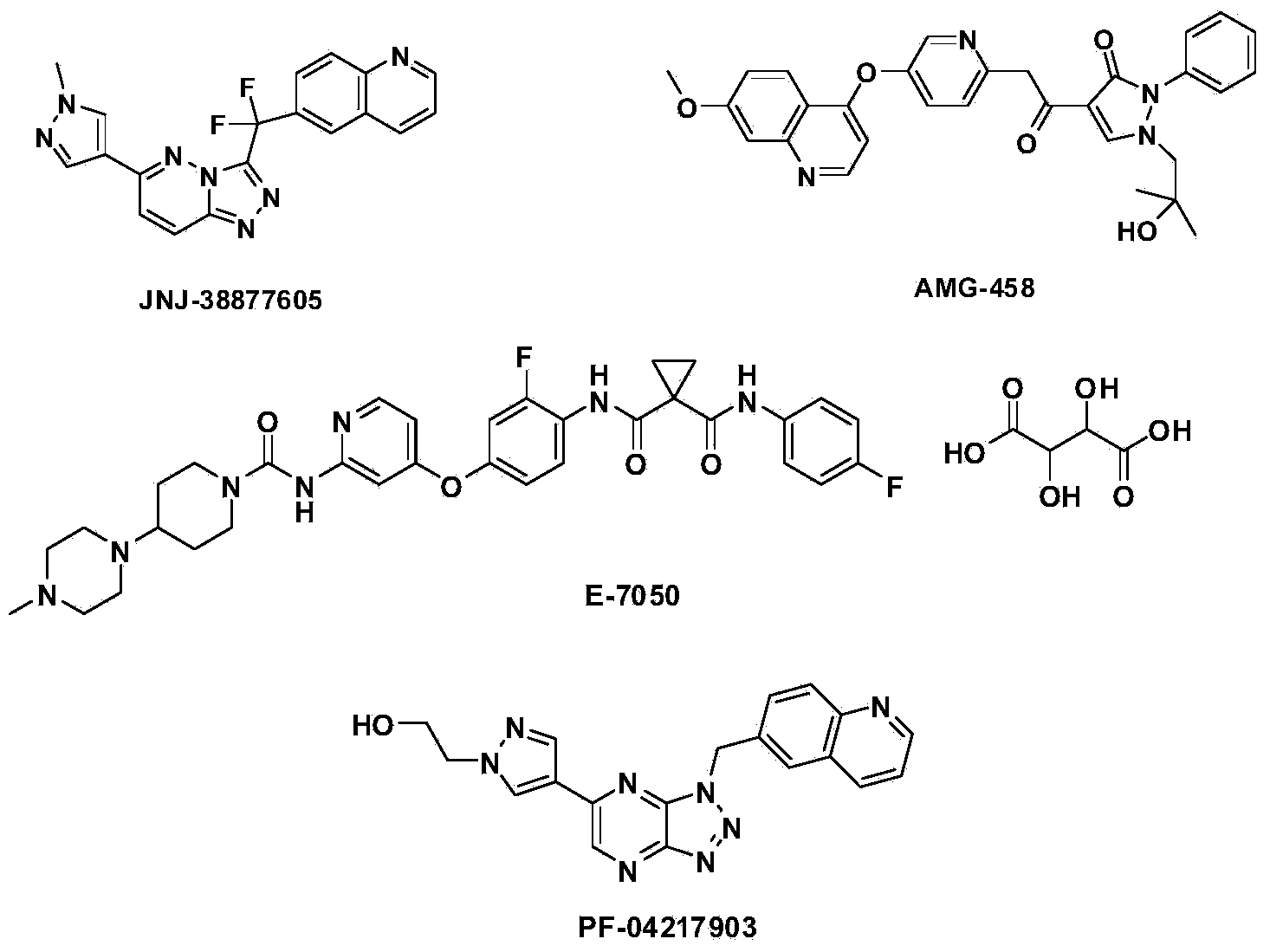
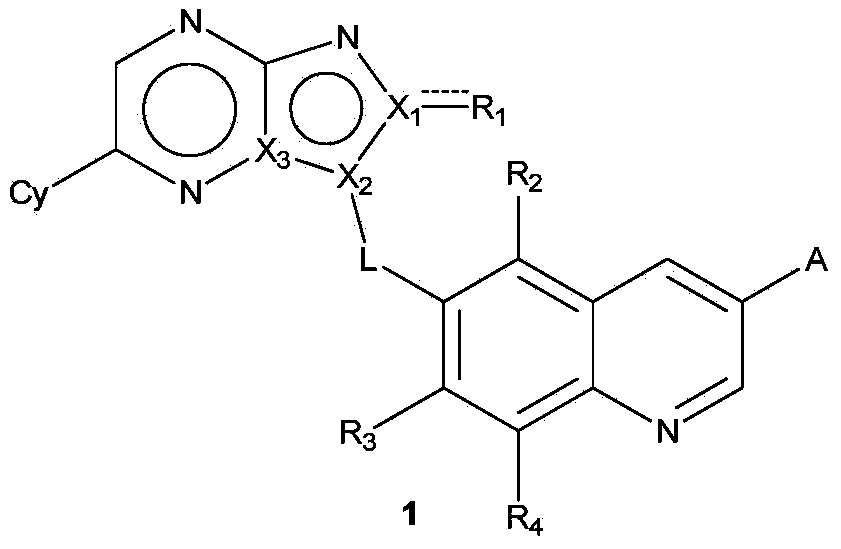
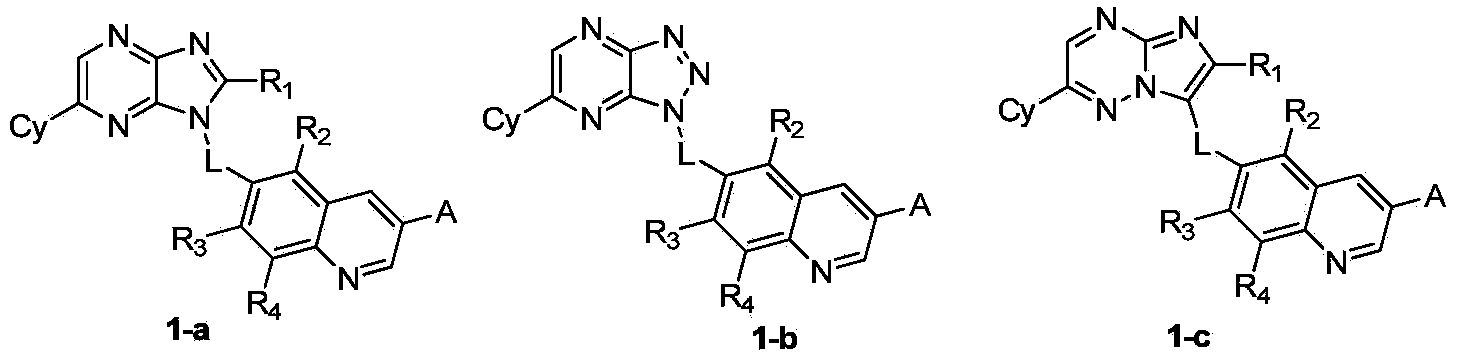
Quinoline compound, and preparation method, intermediate, medicinal composition and application thereof
A technology of quinolines and compounds, applied in the field of quinolines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0596] Example 1: 6-((6-(1-methyl-1H-pyrazol-4-yl)-1H-imidazo[4,5-b]pyrazine-1)-methylene)-quinoline (compound 1-1)
[0597]
[0598] Step 1 Preparation of 6-Bromo-N 2 -(quinoline-6-methylene)pyrazine-2,3-diamine
[0599] 3,5-Dibromopyrazin-2-amine (6.1 g, 24 mmol), 6-quinoline methyleneamine (3.8 g, 24 mmol) and N,N-diisopropylethylamine (DIPEA) (8.6 mL, 48mmol) was added into NMP (20mL), and reacted overnight at 130°C under the protection of argon. The reaction solution was evaporated to remove DIPEA, the residue was poured into water (100mL), extracted with dichloromethane (30mL×3), the organic phase was washed with water, washed with saturated brine, dried and subjected to flash column chromatography to obtain 4.7g of a brown product, yield: 59%. LC-MS (ESI): [M+H] + =330; 1 H-NMR (δppm, DMSO-d 6 ,400MHz): 8.85(dd,J 1 =4.2Hz,J 2 =1.6Hz,1H), 8.33(d,J=8.3Hz,1H), 7.99(d,J=8.7Hz,1H), 7.87(s,1H), 7.73(dd,J 1 =8.7Hz,J 2 =1.9Hz,1H), 7.50(dd,J 1 =8.3Hz,J 2 =4.2Hz, ...
Embodiment 2~ Embodiment 18
[0605] According to the same method as in Example 1, different boric acids or boric acid esters were used to prepare the compounds of Examples 2 to 18, and the specific experimental data are shown in Table 1.
[0606] Table 1 Experimental data table of compound 1-2~compound 1-18
[0607]
[0608]
[0609]
[0610]
[0611]
[0612]
Embodiment 19
[0613] Example 19: 6-(1-(6-(1-methyl-1H-pyrazol-4-yl)-1H-imidazo[4,5-b]pyrazin-1-yl)ethyl)quinone Phenyl (compounds 1-19)
[0614]
[0615] Step 1 Preparation of 6-Bromo-N 2 -(1-(6-quinolyl)ethyl)pyrazine-2,3-diamine
[0616] With reference to the method of Example 1 step 1, 1-(quinolin-6-yl)ethylamine is substituted for 6-quinoline methyleneamine to obtain 6-bromo-N 2 -(1-(6-Quinolinyl)ethyl)pyrazine-2,3-diamine, dark brown solid, yield 68%. LC-MS (ESI): [M+H] + =344; 1 H-NMR (δppm, DMSO-d 6 ,400MHz): 8.85(dd,J 1 =4.2Hz,J 2 =1.7Hz,1H), 8.34(dd,J 1 =8.4Hz,J 2 =0.9Hz,1H), 7.99(d,J=8.7Hz,1H), 7.89(d,J=1.7Hz,1H), 7.79(dd,J 1 =8.7Hz,J 2 =2.0Hz,1H), 7.50(dd,J 1 =8.3Hz,J 2 =4.2Hz,1H), 7.16(s,1H), 7.01(d,J=7.1Hz,1H), 6.35(s,2H), 5.77(d,J=3.3Hz,1H), 5.26(q,J =6.9Hz,1H), 3.36(s,1H), 3.29(dd,J 1 =8.8Hz,J 2 =5.3Hz,1H), 2.53-2.46(m,1H), 2.17(t,J=8.1Hz,1H), 1.95-1.82(m,1H), 1.58(d,J=6.9Hz,3H).
[0617]Step 2 Preparation of 6-(1-(6-bromo-1H-imidazo[4,5-b]pyrazin-1-yl)eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com