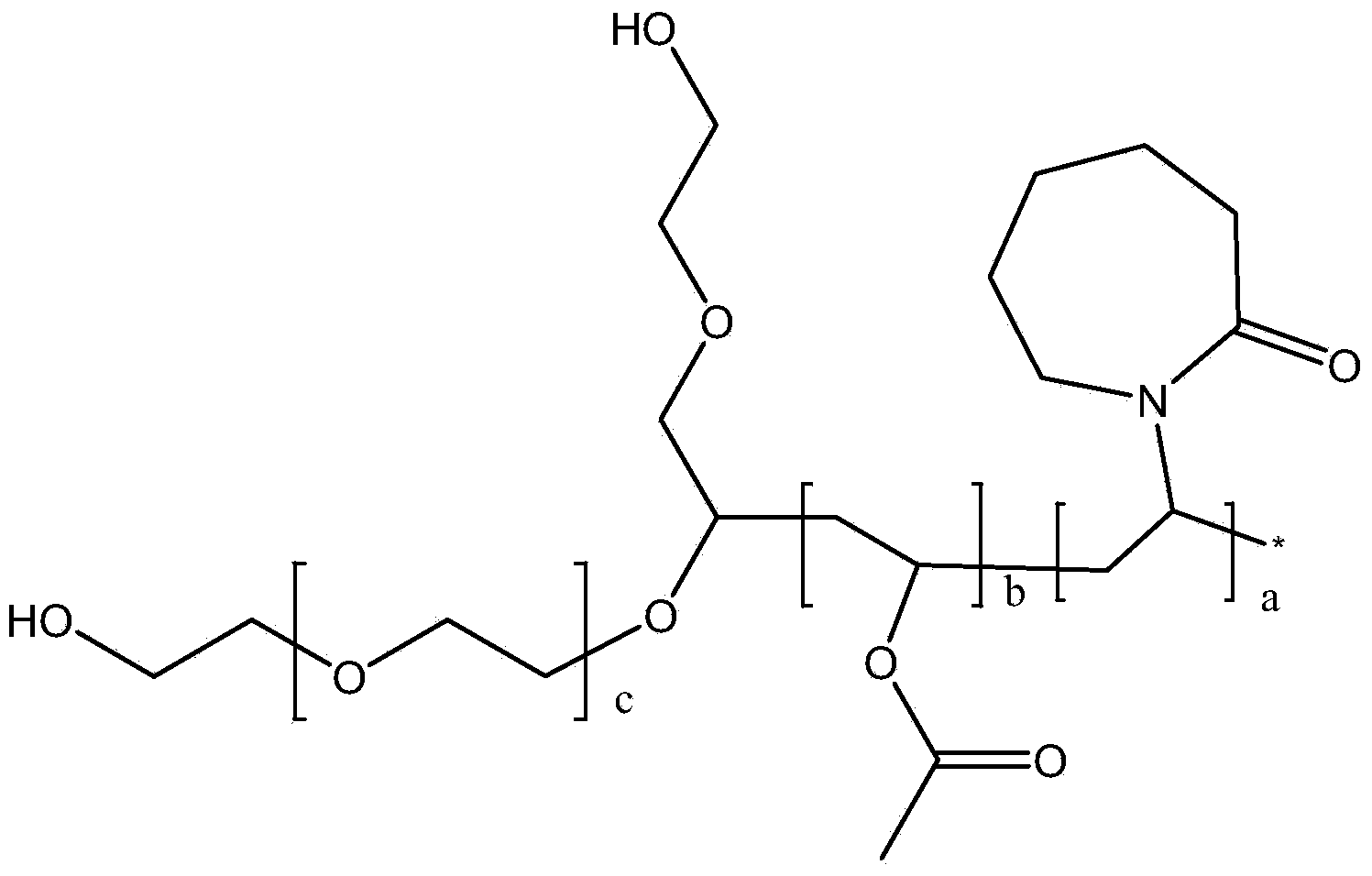
Ophthalmic compositions comprising polyvinyl capralactam-polyvinyl acetate-polyethylene glycol graft copolymers
A technology of polyvinyl acetate and polyethylene glycol grafting, which can be used in drug combinations, medical preparations containing active ingredients, and medical preparations with non-active ingredients, etc., and can solve problems such as poor solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
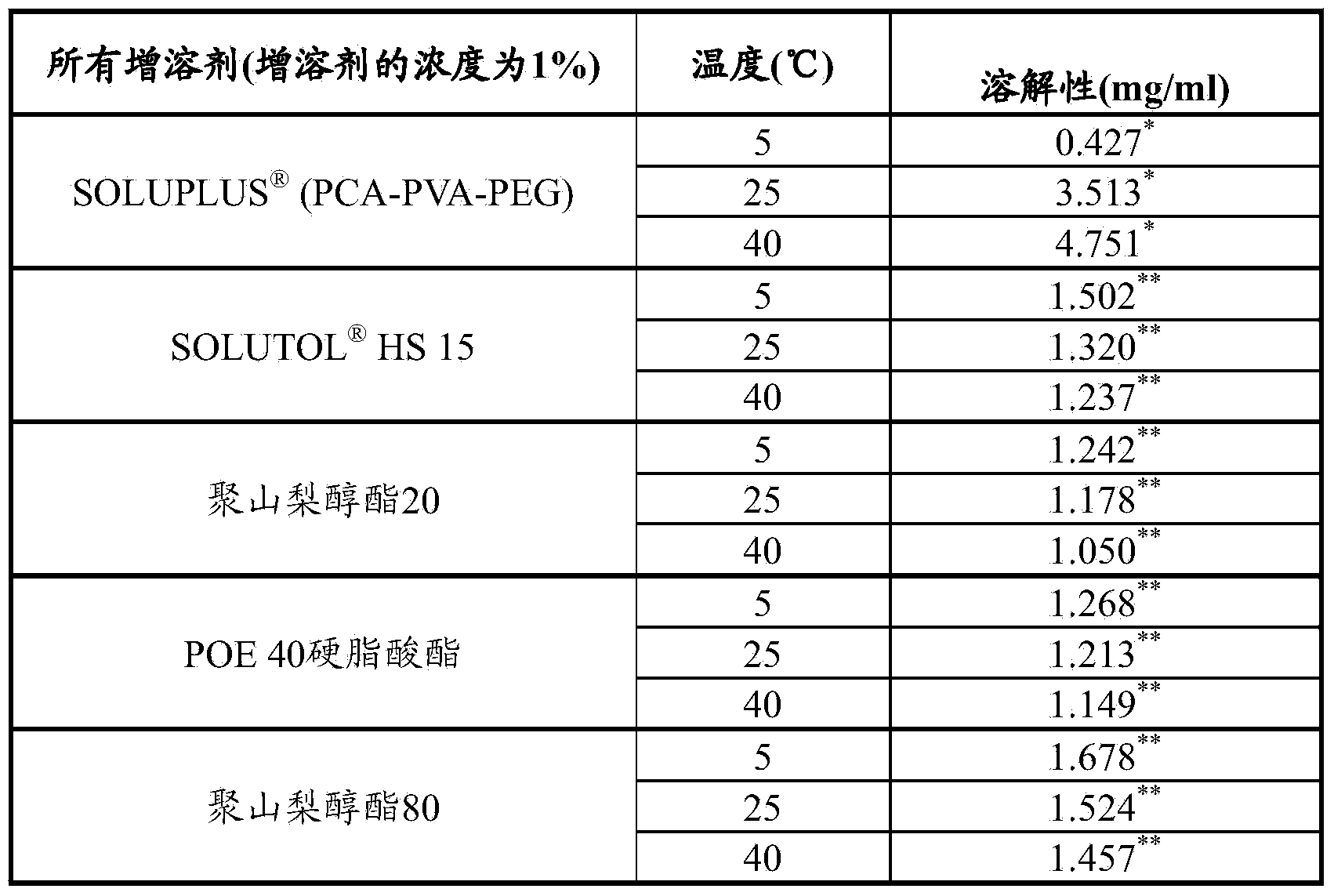
[0080]Table 1 summarizes the solubility of bimatoprost in vehicles with 5 different solubilizers within a certain stability range. Bimatoprost was more soluble in PCA-PVA-PEG containing vehicles than other solubilizers at room temperature (RT) and higher temperatures.
[0081] Table 1 : Solubility of bimatoprost in 5 different formulation vehicles at different temperatures. PCA-PVA-PEG showed the highest solubilization of bimatoprost at room temperature and higher temperature.
[0082]
[0083] * Measured only at week 1; ** Measured at week 8
Embodiment 2
[0085] Data Supporting Improved Efficacy of BAK
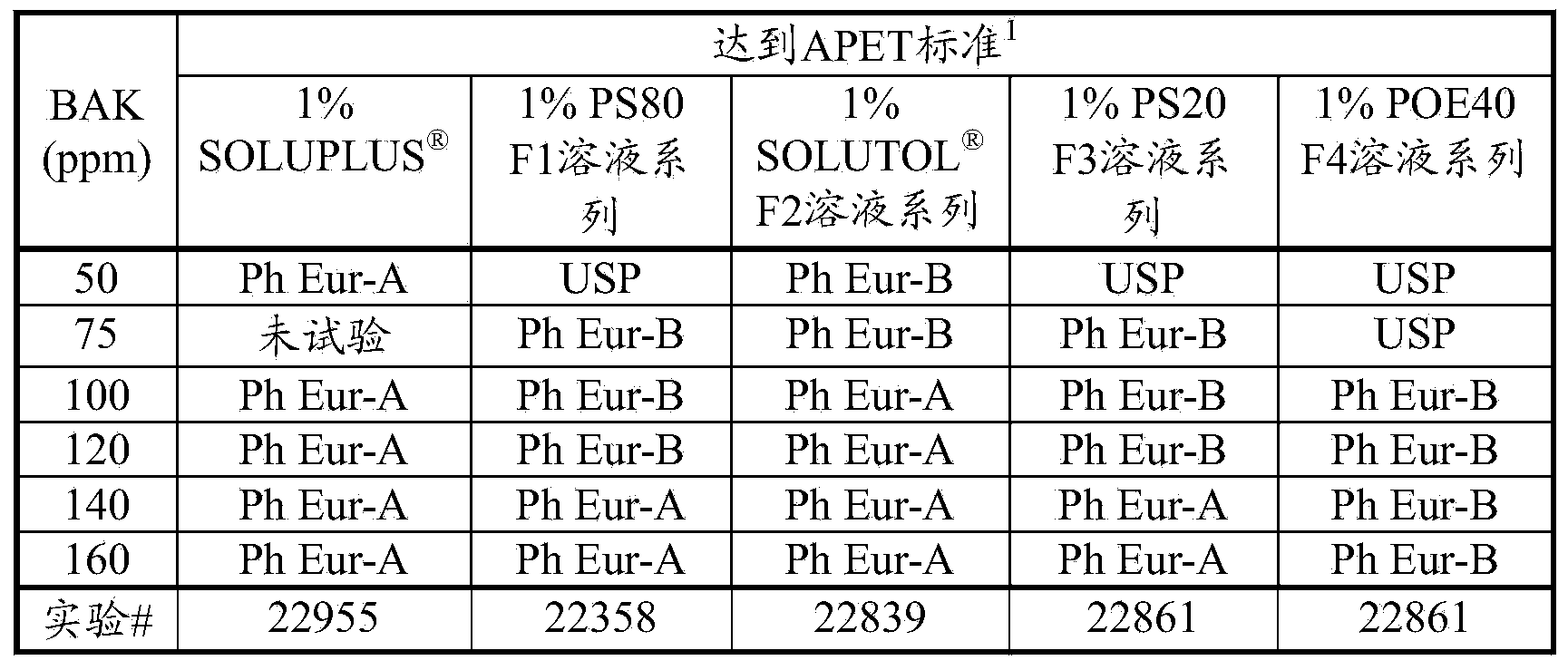
[0086] A preservative titration study was performed to compare the efficacy of BAK in formulations using different solubilizers. In general, it is seen that the preservative efficacy of BAK is significantly reduced in the presence of surfactants. Therefore, higher levels of BAK may be required to meet the standards for preservatives in ophthalmic products as defined in USP and European Pharmacopoeia Chapter 5.1.3. It appears that when using PCA-PVA-PEG as a solubilizer, the preservative criteria are met at lower BAK levels compared to all other surfactants summarized in Table 2. In fact, formulations containing PCA-PVA-PEG met PhEurA criteria with as low as 50 ppm BAK, which was similar to formulations without solubilizers.
[0087] Table 2: Summary of preservative titration to failure results for formulations containing different solubilizers
[0088]
[0089] 1 APET criteria are as defined in USP and European Pharm...
Embodiment 3
[0090] Embodiment 3: the purposes in PCA-PVA-PEG self-corrosion system
[0091] Formulations containing PCA-PVA-PEG exhibited antibacterial activity even without the use of any preservatives. The formulations evaluated are listed in Table 3 along with the APET test results. The formulation containing 1% PCA-PVA-PEG and phosphate-citrate buffer (Formulation 1) was found to meet the USP criteria for all organisms. Phosphate buffer exchange to borate buffer and removal of EDTA brought formulations (Formulations 3-5) to PhEurB standards for all organisms at PCA-PVA-PEG concentrations of 0.5%-1%.
[0092] Table 3: Effect of PCA-PVA-PEG Levels and Borate Buffer in APET Assay
[0093]
[0094]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com