Pharmaceutical composition composed of hydroxamic acid compounds and vitamin A compounds and application thereof
The technology of a compound and hydroxime, which is applied to a pharmaceutical composition composed of a hydroxamic acid compound and a vitamin A acid compound and its application field, can solve the problems of patient pain, toxicity, adverse side effects of normal cells and the like, and achieves the Injury reduction, cost saving, and effect of reducing drug dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
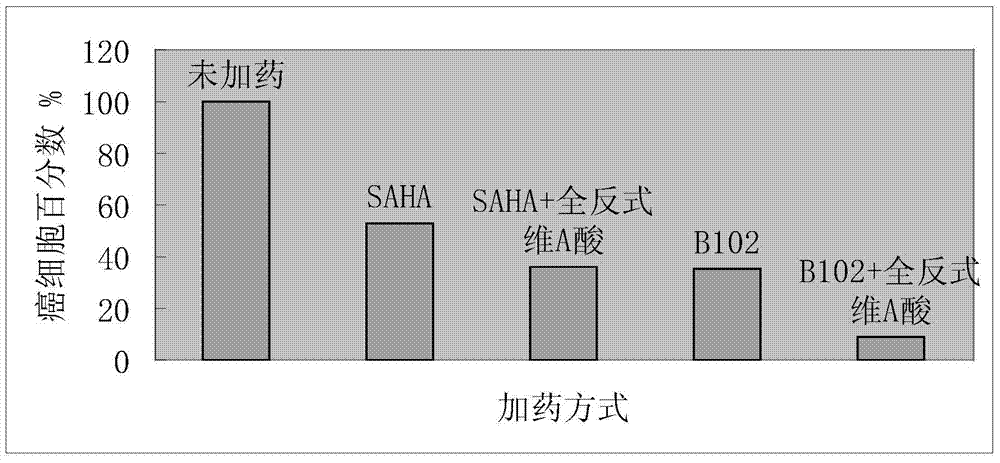
[0025] Preparation of N-(3-chlorophenyl)-N'-hydroxysuberamide (abbreviated as B102).
[0026] Step (1): Weigh 2.04g (16mmol) of 3-chloroaniline, 3.07g (16.32mmol) of monomethyl suberate, and 0.25g (4mmol) of boric acid into a 100mL three-necked round-bottomed flask, add 30mL of toluene, and protect it under nitrogen , magnetically stirred, the oil bath was heated until the toluene refluxed, and the water generated during the reaction was separated with a water separator. Before the reaction, the water separator was filled with toluene, and the reaction time was 18h. The agent is ether:petroleum ether=2:1. After the reaction is completed, pour the reaction solution into 500mL of petroleum ether under stirring, and after stirring for 0.5h, vacuum filter the solid with a sand core funnel, and wash the solid with water to remove the catalyst boric acid, then drain it and wash it with petroleum ether solid and drain the solid. Finally, 3.40g of solid was obtained, and the yield w...
Embodiment 2
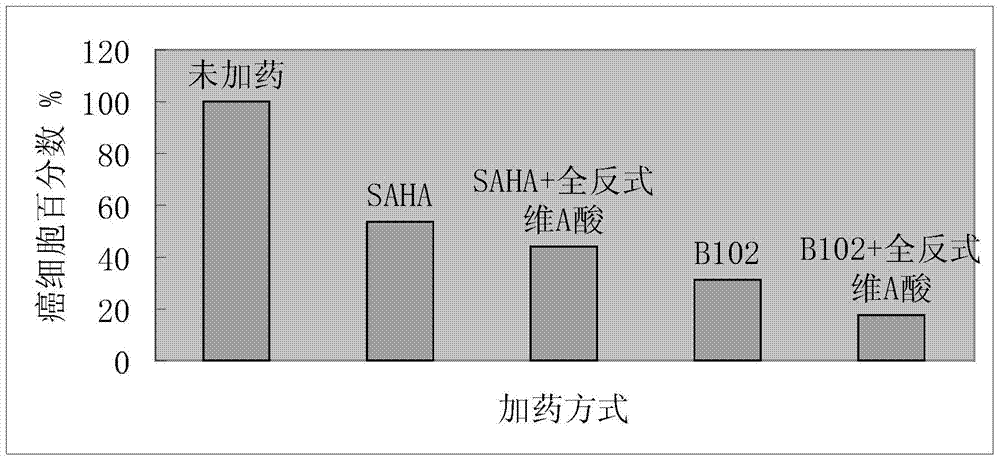
[0029] Preparation of N-(3,4-dimethylphenyl)-N'-hydroxysuberamide (abbreviated as B113).
[0030] Step (1): Weigh 1.21g (10mmol) of 3,4-dimethylaniline, 1.88g (10mmol) of monomethyl suberate, and 0.31g (5mmol) of boric acid in a 100mL three-necked round-bottomed flask, and add 30mL of toluene , nitrogen protection, magnetic stirring, heating the oil bath to toluene reflux, using a water separator to separate the water generated in the reaction process, filling the water separator with toluene before the reaction, the reaction time is 10h, and tracking with a thin layer chromatography plate during the reaction For the reaction, the developer is ethyl acetate:petroleum ether=1:1. After the reaction is completed, pour the reaction solution into 300mL of petroleum ether under stirring. After stirring for 0.5h, vacuum filter the solid with a sand core funnel, and wash the solid with water to remove the catalyst boric acid, and then wash it with petroleum ether after draining. soli...
Embodiment 3
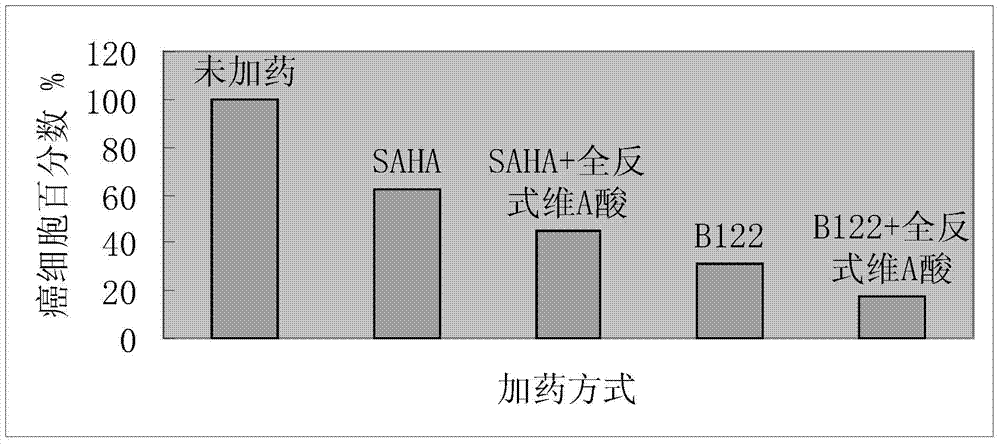
[0033] Preparation of N-(3-trifluoromethoxyphenyl)-N'-hydroxysuberamide (referred to as B124).
[0034] Step (1): Weigh 0.89g (5mmol) of 3-trifluoromethoxyaniline, 0.96g (5.1mmol) of monomethyl suberate, and 0.16g (2.5mmol) of boric acid in a 100mL three-necked round-bottomed flask, and add 30mL toluene, nitrogen protection, magnetic stirring, oil bath heating to toluene reflux, use a water separator to separate the water generated during the reaction, fill the water separator with toluene before the reaction, the reaction time is 13h, and use thin layer chromatography during the reaction Plate tracking reaction, the developer is ether: petroleum ether = 1:1. After the reaction is completed, pour the reaction solution into 200mL of petroleum ether under stirring, and after stirring for 0.5h, vacuum filter the solid with a sand core funnel, and wash the solid with water to remove the catalyst boric acid, then drain it and wash it with petroleum ether solid and drain the solid....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com