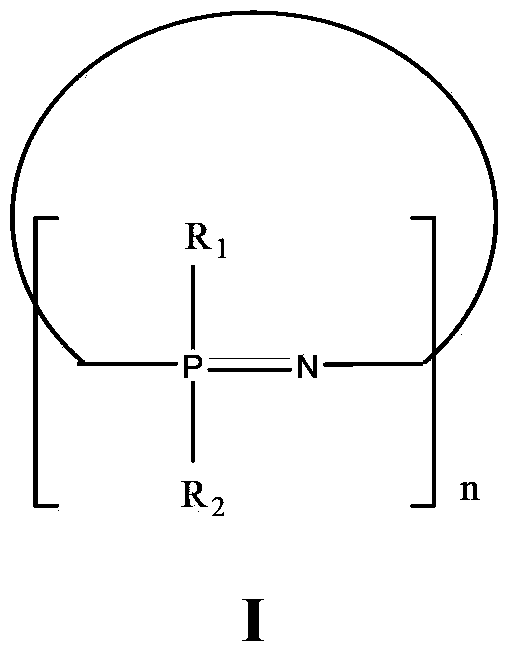
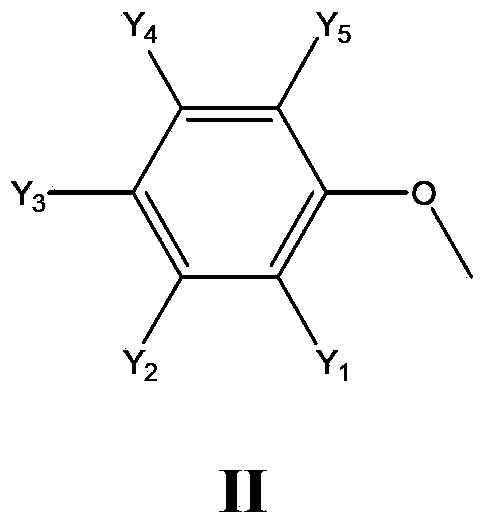
Application of cyclophosphazene compound in preparation of resin as antidrip agent
A technology of cyclic phosphazenes and anti-dripping agents, applied in the field of polymers, can solve problems not related to the application of anti-dripping agents of cyclic phosphazenes, achieve excellent anti-dripping performance, improve flame retardancy, and excellent Effect of mechanical properties and processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
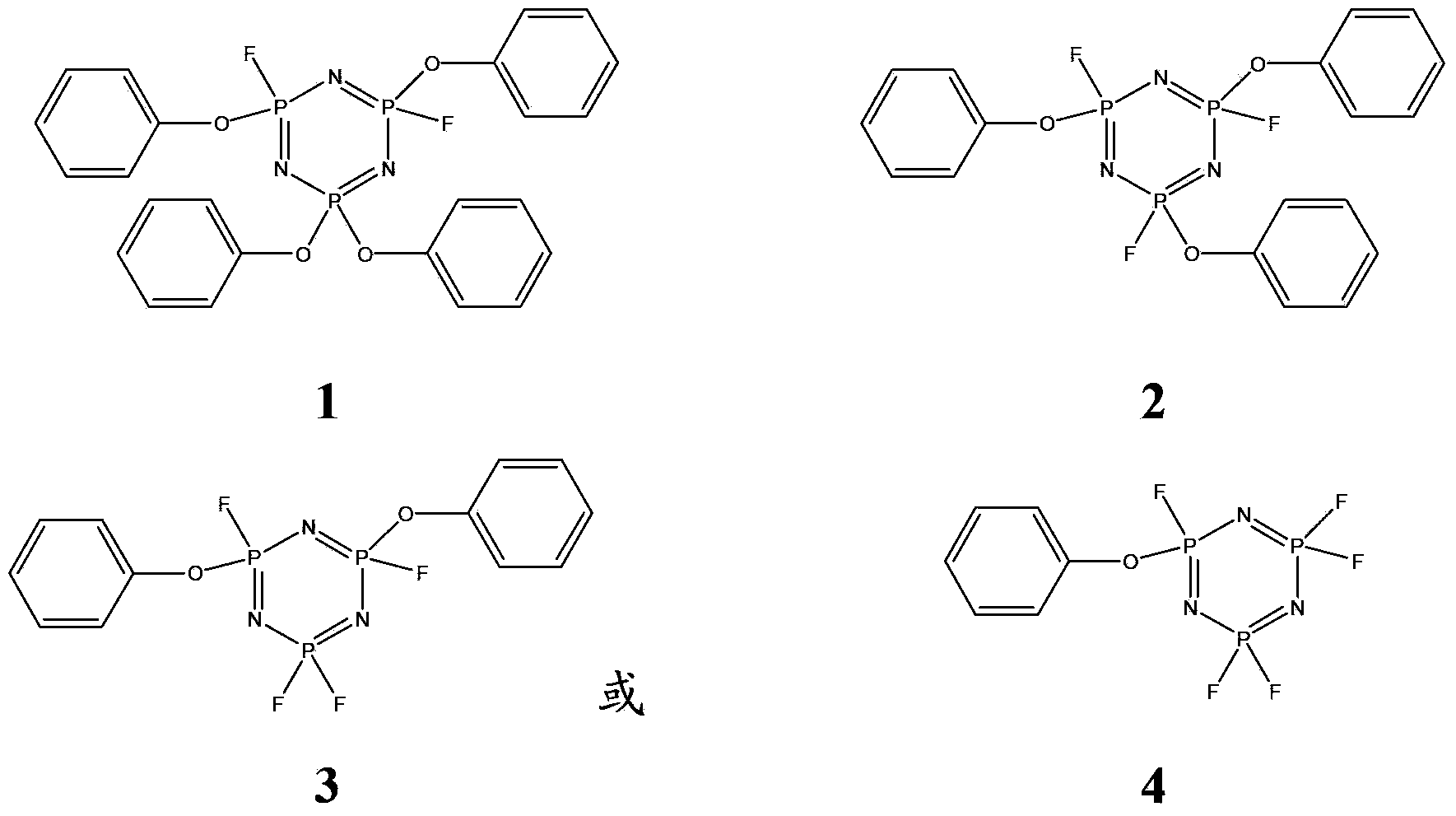
[0090] Synthesis of compound 2:
[0091]
[0092] Dissolve 34.8g (0.1mol) of hexachlorocyclotriphosphazene in 175mL of chlorobenzene solvent, heat to 30°C, and then add 27.3g (0.65mol) of sodium fluoride for fluorination to obtain hexafluorocyclotriphosphazene . Slowly add phenol tetrahydrofuran solution (equivalent to phenol 29.3 g, 0.31 mol) into the hexafluorocyclotriphosphazene solution at 25°C, and use sodium hydroxide as an acid-binding agent to react for 30 minutes. Filter to remove the inorganic salts and unreacted bases generated by the reaction. The filtrate is evaporated under reduced pressure to remove the solvent (recovery and reuse), then add 175 mL of cyclohexane to dissolve, then wash with dilute lye, dilute hydrochloric acid, and 3 times of water to remove impurities. The solvent was removed from the organic layer under reduced pressure (cyclohexane was recovered and reused) to obtain 37.8 g of compound 2 with a yield of 80% and a purity of 99.0%. Boiling...
Embodiment 2
[0094] Synthesis of compound 3:
[0095]
[0096] Dissolve 34.8g (0.1mol) of hexachlorocyclotriphosphazene in 175mL of chlorobenzene solvent, heat to 30°C, and then add 27.3g (0.65mol) of sodium fluoride for fluorination to obtain hexafluorocyclotriphosphazene . Slowly add phenol acetonitrile solution (equivalent to 19.3 g of phenol, 2.05 mol) into the hexafluorocyclotriphosphazene solution at 25°C, while using sodium hydroxide as an acid-binding agent, and react for 30 minutes. Filter to remove the inorganic salts and unreacted bases generated by the reaction. The filtrate is evaporated under reduced pressure to remove the solvent (recovery and reuse), then add 175 mL of cyclohexane to dissolve, then wash with dilute lye, dilute hydrochloric acid, and 3 times of water to remove impurities. The solvent was removed from the organic layer under reduced pressure (cyclohexane was recovered and reused) to obtain 29.8 g of compound 3 with a yield of 75% and a purity of 98.2%. M...
Embodiment 3
[0098] Synthesis of compound 1:
[0099]
[0100] Dissolve 34.8g (0.1mol) of hexachlorocyclotriphosphazene in 175mL of chlorobenzene solvent, heat to 30°C, and then add 27.3g (0.65mol) of sodium fluoride for fluorination to obtain hexafluorocyclotriphosphazene . Slowly add phenol tetrahydrofuran solution (equivalent to phenol 39.7 g, 0.42 mol) into the hexafluorocyclotriphosphazene solution at 25°C, while using sodium carbonate as an acid-binding agent, and react for 3 hours. Add water to dissolve the inorganic salt and unreacted base, filter, wash with base, acid, and water three times in sequence, and dry the filter cake to obtain 45.5 g of compound 1 with a yield of 83.5% and a purity of 99.3%. MS (ESI) m / z: 546.35 [M+H].
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com