Preparation method of loading type bimetallic catalyst with highly-dispersing active center
A metal catalyst and active center technology, which is applied in the control field of uniform dispersion of supported catalyst metal centers and auxiliary metals, can solve the problems of difficult realization, difficult operation, and reduced active center amount, and achieves uniform dispersion, high dispersion and high isomerism. selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
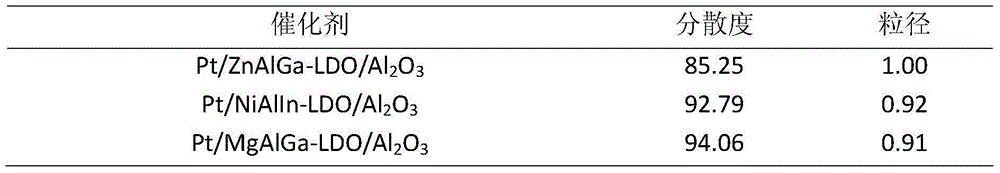
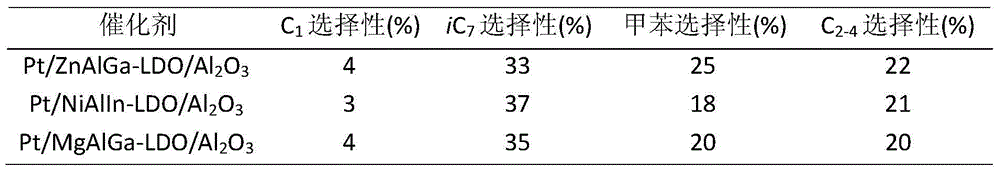
[0025] Zinc sulfate, gallium nitrate, and hexamethylenetetramine were dissolved in deionized water to form solution A. Dip aluminum oxide into solution A, transfer it to a high-pressure reactor, and crystallize at 130°C for 6 hours. The molar ratio of zinc sulfate, gallium nitrate, hexamethylene tetramine and aluminum oxide is 150:8:800. After the reaction, the reaction solution was suction-filtered, washed until the eluent was neutral, and the obtained hydrotalcite-modified aluminum oxide was dried at 80° C. for 12 hours. The high-valent metals required by the hydrotalcite laminates are supplemented by the aluminum dissolved by the aluminum oxide during the reaction process.
[0026] Step B: Dissolve platinum acetylacetonate in a certain amount of deionized water or other solvents to form solution B, immerse the aluminum oxide modified by hydrotalcite obtained in step (1) in solution B, let it stand for 12h, and then Dry at 80°C for 12h. The molar ratio of platinum acetyla...
Embodiment 2
[0031] Nickel nitrate, indium nitrate and ammonia water were dissolved in deionized water to form solution A. Dip aluminum oxide into solution A, transfer it to a high-pressure reactor, and crystallize at 160°C for 2 hours. The molar ratio of nickel nitrate, indium nitrate, hexamethylene tetramine and aluminum oxide is 50:5:50:1500. After the reaction, the reaction solution was suction-filtered, washed until the eluent was neutral, and the obtained hydrotalcite-modified aluminum oxide was dried at 80° C. for 12 hours. The high-valent metals required by the hydrotalcite laminates are supplemented by the aluminum dissolved by the aluminum oxide during the reaction process.
[0032] Step B: Dissolve sodium chloroplatinate in a certain amount of deionized water or other solvents to form solution B, immerse the hydrotalcite-modified aluminum oxide obtained in step (1) in solution B, let stand for 12h, and then Dry at 80°C for 12h. Wherein the molar ratio of sodium chloroplatinat...
Embodiment 3
[0037] Step A: Combine Magnesium Nitrate with Urea
[0038] Magnesium nitrate, gallium nitrate, and urea were dissolved in deionized water to form solution A. Dip aluminum oxide into solution A, transfer it to a high-pressure reactor, and crystallize at 80°C for 12 hours. The molar ratio of magnesium nitrate, gallium nitrate, urea and aluminum oxide is 30:1:200:100. After the reaction, the reaction solution was suction-filtered, washed until the eluent was neutral, and the obtained hydrotalcite-modified aluminum oxide was dried at 80° C. for 12 hours. The high-valent metals required by the hydrotalcite laminates are supplemented by the aluminum dissolved by the aluminum oxide during the reaction process.
[0039] Step B: Hexaaminoplatinum nitrate is dissolved in a certain amount of deionized water or other solvents to form solution B, and the hydrotalcite-modified Al obtained in step (1) 2 o 3 Immerse in solution B, let stand for 12h, then dry at 80°C for 12h. The molar r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com