Doping type supercapacitor electrode material
A technology for supercapacitors and electrode materials, which is applied in the direction of hybrid capacitor electrodes, etc., can solve the problems of poor cycle performance, power density, and low energy density, and achieve the effects of easy large-scale application, excellent rate performance, and simple and effective methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) Mix 0.166g terephthalic acid, 0.096g nickel nitrate hexahydrate and 0.0228g ZnCl 2 Dissolve in 20 ml DMF;
[0023] 2) Dissolve 1.6g NaOH in 100ml H 2 O middle;
[0024] 3) Add 2ml of the solution described in step 2) dropwise to the solution described in step 1) at a rate of 1ml / min;
[0025] 4) Put the solution described in step 3) into the reactor, and react at 100°C for 8 hours;
[0026] 5) The product obtained in step 4) was washed three times with DMF and ethanol respectively, and baked at 70°C for 24 hours to obtain a sample;
[0027] 6) The zinc-doped nickel-based metal-organic framework material is made into a supercapacitor electrode through steps such as wet mixing, rolling into a film, and drying.
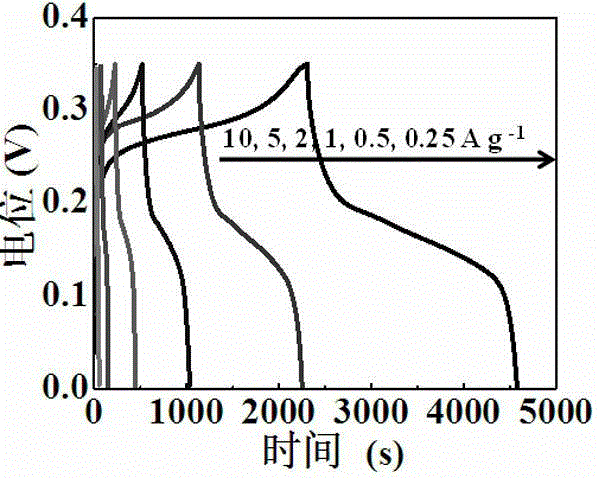
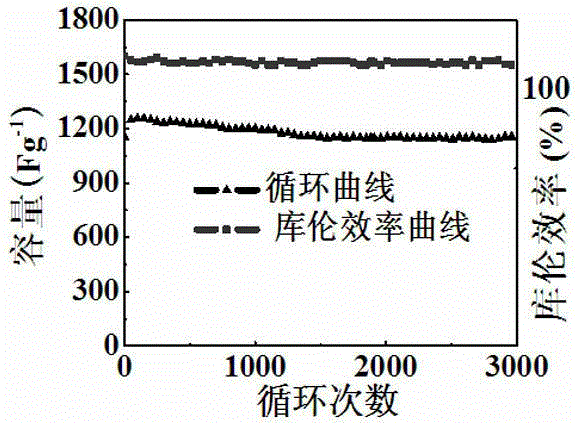
[0028] The zinc-doped nickel-based metal-organic framework electrode prepared in this example has a maximum specific capacity of 1620 F g -1 , after 3000 cycles, the capacity retention rate is 91%.
Embodiment 2
[0030] 1) Mix 0.166g terephthalic acid, 0.096g nickel nitrate hexahydrate and 0.0456 ZnCl 2 Dissolve in 20 ml DMF;
[0031] 2) Dissolve 1.6g NaOH in 100ml H 2 O middle;
[0032] 3) Add 2ml of the solution described in step 2) dropwise to the solution described in step 1) at a rate of 1ml / min;
[0033] 4) Put the solution described in step 3) into the reactor, and react at 100°C for 8 hours;
[0034] 5) The product obtained in step 4) was washed three times with DMF and ethanol respectively, and baked at 70°C for 24 hours to obtain a sample;
[0035] 6) The zinc-doped nickel-based metal-organic framework material is made into a supercapacitor electrode through steps such as wet mixing, rolling into a film, and drying.
[0036] The zinc-doped nickel-based metal-organic framework electrode prepared in this example has a maximum specific capacity of 1630 F g -1 , after 3000 cycles, the capacity retention rate is 91.2%.
Embodiment 3
[0038] 1) Mix 0.166g terephthalic acid, 0.096g nickel nitrate hexahydrate and 0.0912g ZnCl 2 Dissolve in 20 ml DMF;
[0039] 2) Dissolve 1.6g NaOH in 100ml H 2 O middle;
[0040] 3) Add 2ml of the solution described in step 2) dropwise to the solution described in step 1) at a rate of 1ml / min;
[0041] 4) Put the solution described in step 3) into the reactor, and react at 100°C for 8 hours;
[0042] 5) The product obtained in step 4) was washed three times with DMF and ethanol respectively, and baked at 70°C for 24 hours to obtain a sample;
[0043] 6) The zinc-doped nickel-based metal-organic framework material is made into a supercapacitor electrode through steps such as wet mixing, rolling into a film, and drying.
[0044] The zinc-doped nickel-based metal-organic framework electrode prepared in this example has a maximum specific capacity of 1615 F g -1 , after 3000 cycles, the capacity retention rate is 90.3%.
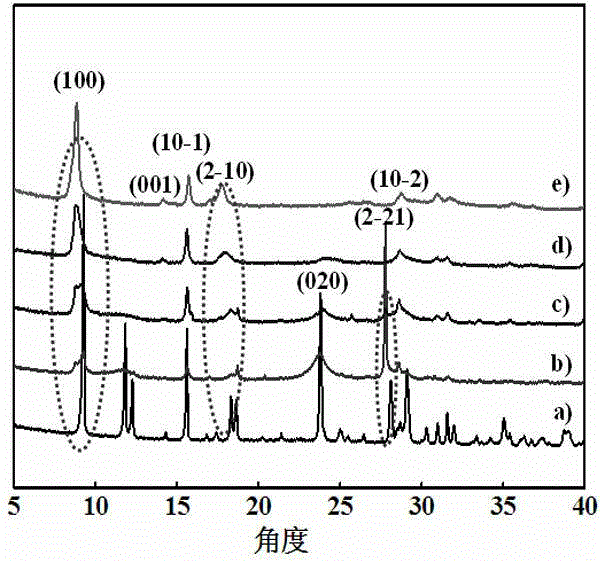
[0045] figure 1 It is an X-ray diffraction pattern o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com