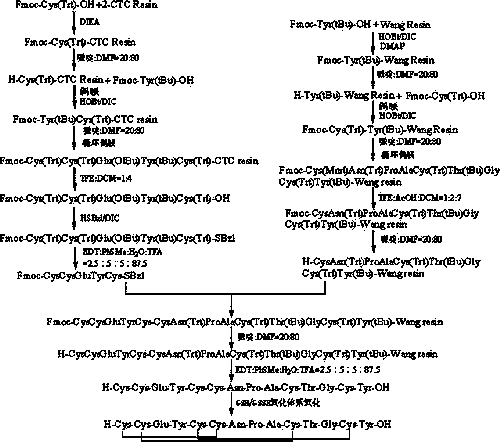
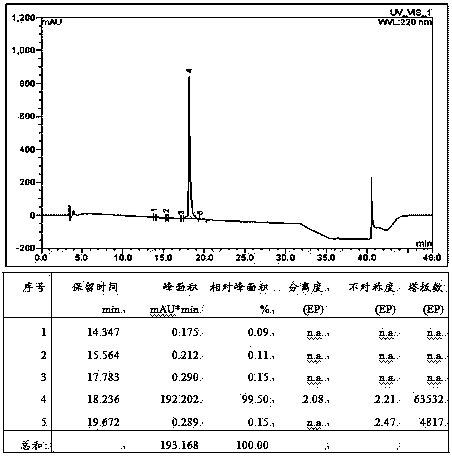
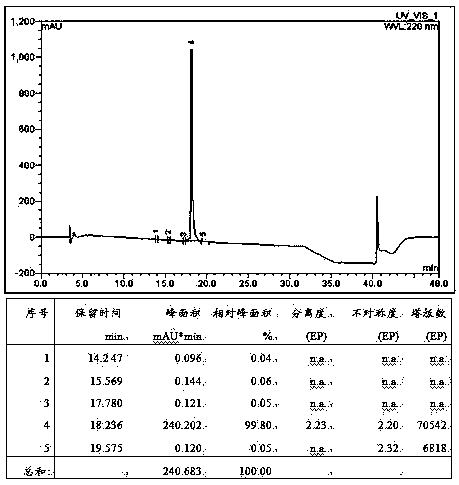
Method for preparing linaclotide
A technology of linaclotide and a synthesis method, which is applied in the field of peptide medicine synthesis, can solve the problems of many impurities, high cost, unfavorable large-scale production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment one: the synthesis of the Fmoc-Cys(Trt)-CTC resin that the degree of substitution is 0.10mmol / g
[0086] Weigh 50.00g of 2-CTC resin with a substitution degree of 0.40mmol / g, add it to the solid phase reaction column, add it to the solid phase reaction column, wash once with DMF, swell the resin with DMF for 30 minutes, and take 58.57g Fmoc-Cys(Trt)-OH (100mmol) was dissolved in DMF, activated by adding 17ml DIEA (100mmol) in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, reacted for 2 hours, added 500ml of anhydrous methanol to seal for 1 hour . Wash 3 times with DMF and 3 times with DCM, block with anhydrous methanol for 30 minutes, shrink and dry with methanol to obtain Fmoc-Cys(Trt)-CTC resin, the detection degree of substitution is 0.10mmol / g.
Embodiment 2
[0087] Embodiment two: the degree of substitution is the synthesis of Fmoc-Cys(Trt)-CTC resin of 0.90mmol / g
[0088] Weigh 133.33g of 2-CTC resin with a degree of substitution of 1.50mmol / g, add it to the solid phase reaction column, add it to the solid phase reaction column, wash once with DMF, swell the resin with DMF for 30 minutes, and take 585.70g Fmoc-Cys(Trt)-OH (1000mmol) was dissolved in DMF, activated by adding 165ml DIEA (1000mmol) in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, reacted for 2 hours, added 2000ml of anhydrous methanol to block for 1 hour . Wash 3 times with DMF and 3 times with DCM, block with anhydrous methanol for 30 minutes, shrink and dry with methanol to obtain Fmoc-Cys(Trt)-CTC resin, the detection degree of substitution is 0.90mmol / g.
Embodiment 3
[0089] Embodiment three: the degree of substitution is the synthesis of Fmoc-Cys(Trt)-CTC resin of 0.50mmol / g
[0090] Weigh 200.00g of 2-CTC resin with a degree of substitution of 1.00mmol / g, add it to the solid phase reaction column, add it to the solid phase reaction column, wash once with DMF, swell the resin with DMF for 30 minutes, and take 585.70g Fmoc-Cys(Trt)-OH (1000mmol) was dissolved in DMF, activated by adding 165ml DIEA (1000mmol) in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, reacted for 2 hours, added 2000ml of anhydrous methanol to block for 1 hour . Wash 3 times with DMF and 3 times with DCM, block with anhydrous methanol for 30 minutes, shrink and dry with methanol to obtain Fmoc-Cys(Trt)-CTC resin, the detection degree of substitution is 0.50mmol / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com