Method for recovering iron from sulphuric acid leachate through extraction
A technology for leaching liquid and recovering iron, which is applied in the direction of improving process efficiency, etc., can solve problems such as complicated operation, secondary pollution, and being in the experimental stage, and achieve the effects of simple operation, avoiding consumption, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
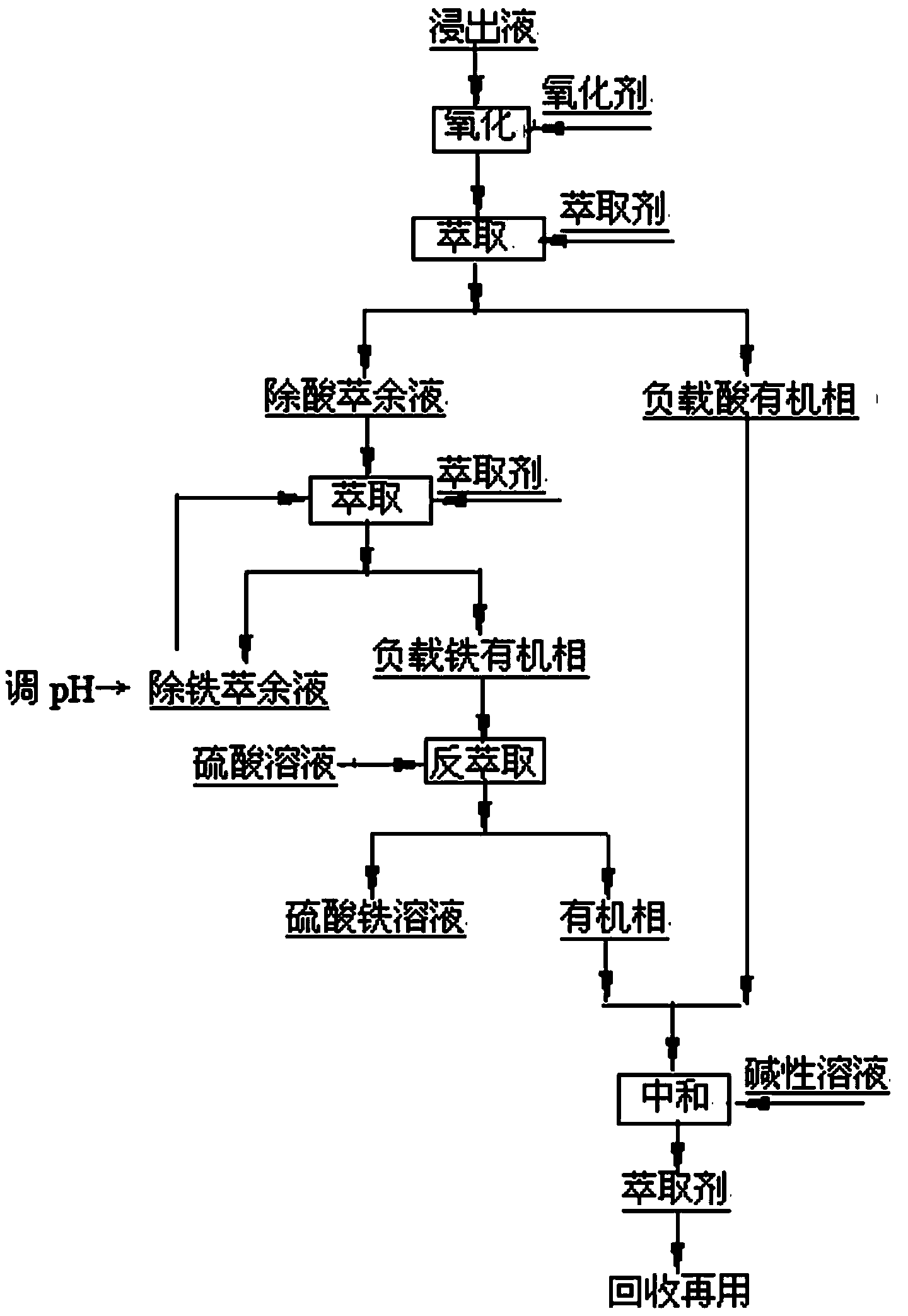
[0018] The sulfuric acid leaching solution of a composite ore contains Fe 14.0g / L (of which Fe 2+ 2.8g / L), Cu 5.35g / L, Co 0.25g / L, Ni 6.31g / L, the concentration of free acid is 0.32mol / L, add hydrogen peroxide which is 1.2 times the theoretical amount required for the oxidation of ferrous iron into the leaching solution After oxidation, the volume ratio of 20%N235-5%TBP-75% sulfonated kerosene extraction agent and leachate is O:A=1:1, and the extraction time is 2min for a first-stage cross-flow extraction to the acid-removed raffinate pH=0.89, after the first extraction of iron, the raffinate pH=1.68, the pH was adjusted to 0.7, the second extraction of iron, the raffinate pH=1.88, the pH was adjusted to 0.7, the third extraction of iron, the obtained extraction The remaining liquid contains Fe 0.31g / L, and the calculated iron extraction rate is 97.79%. The iron-loaded organic phase and 20g / L sulfuric acid solution are subjected to two-stage countercurrent stripping at O:A=1:...
Embodiment 2
[0020] The sulfuric acid leaching solution of a composite ore has a main component content of Fe 28.4g / L (of which Fe 2+ 4.53g / L), Cu 4.35g / L, Co 0.19g / L, Ni 4.63g / L, the concentration of free acid is 0.78mol / L, add hydrogen peroxide which is 1.2 times the theoretical amount required for the oxidation of ferrous iron into the leaching solution After oxidation, the volume ratio of 40%N235-20%TBP-40% sulfonated kerosene extractant and leachate is O:A=1:1, and the extraction time is 20min to perform a first-stage cross-flow extraction from acid to acid removal raffinate Liquid pH=0.95, after extracting iron for the first time, the raffinate pH=2.34, adjusting the pH to 0.9, extracting iron for the second time, the obtained raffinate contains Fe 0.56g / L, and the calculated iron extraction rate is 98.04%; The iron-loaded organic phase and 60g / L sulfuric acid solution were subjected to a first-stage back extraction according to O:A=1:1, and the back extraction time was 20min. The b...
Embodiment 3
[0022] The iron-containing sulfuric acid leaching solution of a composite ore has a main component content of Fe 28.4g / L (of which Fe 2+ 4.53g / L), Cu 4.35g / L, Co 0.19g / L, Ni 4.63g / L, the concentration of free acid is 0.78mol / L, and 1.5 times the theoretical amount required for the oxidation of divalent iron is added to the leaching solution. After sodium sulfate oxidation, the volume ratio of 30%N235-10%TBP-60% sulfonated kerosene extraction agent and leachate is O:A=1:1, and the extraction time is 2min for 1-stage cross-flow extraction to acid removal extraction. The raffinate pH=0.75, after extracting iron for the first time, the raffinate pH=1.88, adjust the pH to 1.2, extract iron for the second time, the raffinate pH=2.52, adjust the pH to 0.95, extract iron for the third time, extract Raffinate pH=2.24, adjust pH to be 1.2, extract iron for the 4th time, obtained raffinate contains Fe 0.52g / L, calculates iron extraction rate 98.17%; Load iron organic phase and 60g / L sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com