Bromized aliphatic olefin/vinyl aromatic copolymer flame retardant and preparation method thereof
A vinyl aromatic and bromostyrene-based technology, which is used in the field of plastic additives in the chemical industry, can solve the problems of reduced flame retardant performance, environmental hazards, and reduced flame retardant performance of flame retardants, so as to improve the flame retardant performance , good thermal stability and flame retardant properties, the effect of increasing the bromination rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
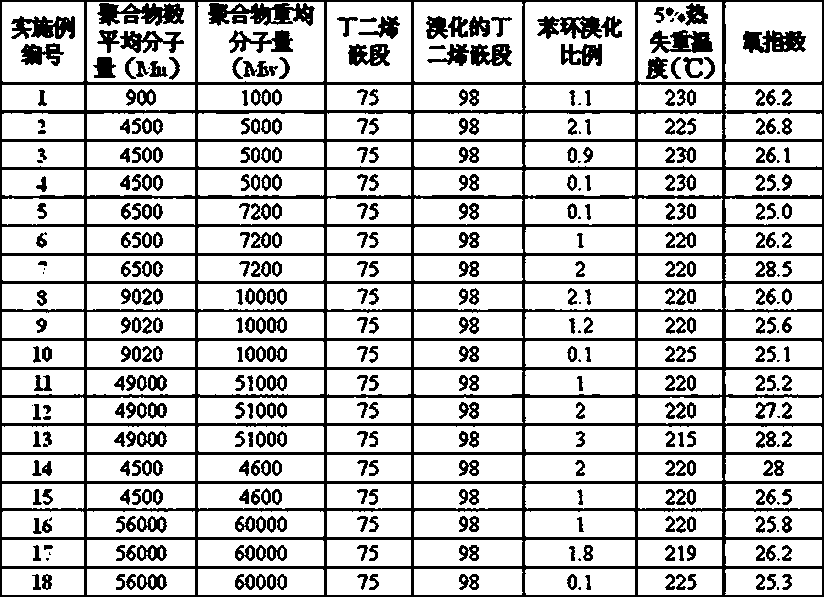
Examples
Embodiment 1
[0038] Add 6.6 g of (polystyrene-b-butadiene) copolymer (0.075 moles of polymerized polybutadiene units) and 70 ml of carbon tetrachloride solution to a tank equipped with a mechanical stirrer and a constant pressure dropping funnel In a 250ml three-necked flask, stir to dissolve evenly, then add 25ml of n-butanol and mix evenly. 12.6 g (0.08 mol) of bromine was added dropwise to the solution at room temperature, and the addition was completed within 20 min. The reaction mixture exhibited an exotherm during the bromine addition with a maximum temperature of 45 °C during the reaction. After the reaction mixture was continued to react at room temperature for 15 hours, 0.01 g of ferric chloride was added, and the reaction was continued for 2 hours, and then 50 ml of 20 wt % sodium bisulfite aqueous solution was added. Stirring was then continued for 15 minutes, after which 60 ml of dichloromethane were added. The reaction mixture was transferred to a separatory funnel with the ...
Embodiment 2
[0041] Add 6.6 g of poly(styrene-b-butadiene) copolymer (0.075 moles of polymerized polybutadiene units) and 70 ml of dichloromethane solution into a 250 ml In a three-necked flask, stir and dissolve evenly, then add 25ml of n-butanol and mix evenly. After the polymer was dissolved, the solution was cooled to 1°C in an ice bath, and 12.6 g (0.008 moles) of bromine was added dropwise to the solution, and the addition was completed within 20 minutes, keeping the temperature in the reaction flask at or below 7°C. After continuing to stir for 20 minutes, add 0.01 g of ferric chloride, continue the reaction for 30 min, remove the ice bath, and use 20% aqueous sodium bisulfite solution (51 g) for the reaction solution, then water (50 g), and then use saturated chlorine Aqueous sodium chloride solution (59 g) was washed, followed by extraction with dichloromethane. The washed organic phase was added to a 5-fold excess (based on solution volume) of methanol, the brominated polymer wa...
Embodiment 3
[0043] Add 6.6 g of poly(styrene-b-butadiene) copolymer (0.075 moles of polymerized polybutadiene units) and 70 ml of dichloromethane solution into a 250 ml In a three-necked flask, stir and dissolve evenly, then add 25ml of n-butanol and mix evenly. After the polymer had dissolved, the solution was cooled to 1 °C in an ice bath. 20 ml of THF solution containing 25.6 g of pyridinium tribromide (0.008 mol) was added dropwise to the solution, and the addition was completed within 30 min. Keep the temperature in the reaction flask at or below 7°C, and after continuing to stir for 20 minutes, add 0.01 g of ferric chloride, continue the reaction for 30 minutes, and remove the ice bath; and the reaction solution is filtered to remove by-product pyridinium hydrobromide ( PHB), the resulting polymer solution was passed through a cation exchange resin bed to remove residual PHB from the solution, followed by 20% aqueous sodium bisulfite (51 g), followed by water (50 g), then saturated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com