Mandelic Acid Condensation Polymer
A technology of compounds and compositions, applied in the field of mandelic acid condensation polymers, which can solve the problem of increased infection risk of the protective skin layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124]
[0125] Mandelic acid condensation polymers of formula (3a) were prepared by direct reaction of dl-mandelic acid with concentrated sulfuric acid at low temperature. At -35 °C, under vigorous stirring, the solution containing concentrated H 2 SO 4(256 grams (g), 4000 millimoles (mmol), 139 ml) of a round bottom flask equipped with a mechanical stirrer was added dl-mandelic acid (30.4 g, 200 mmol). The resulting reaction mixture was stirred at -35°C for 1 hour and then at room temperature for an additional 8 hours. The reaction mixture was then poured into an Erlenmeyer flask containing 3040 ml of ice water and stirred for 1 hour. The solid was isolated and filtered with suction, washed with water (3 x 50ml) and dried in a vacuum desiccator under reduced pressure for 12 hours to give (3a) as an off white solid (28.25g, 100 % yield); melting point (mp) 205-206°C. 1 H NMR (DMSO-d 6 ): δ7.35-7.02(m, ArH), 5.23(br s, CHOH), 5.15-4.75(m, CHCO 2 H), 3.41 (br s, OH). ...
Embodiment 2
[0127] ESI-MS analysis
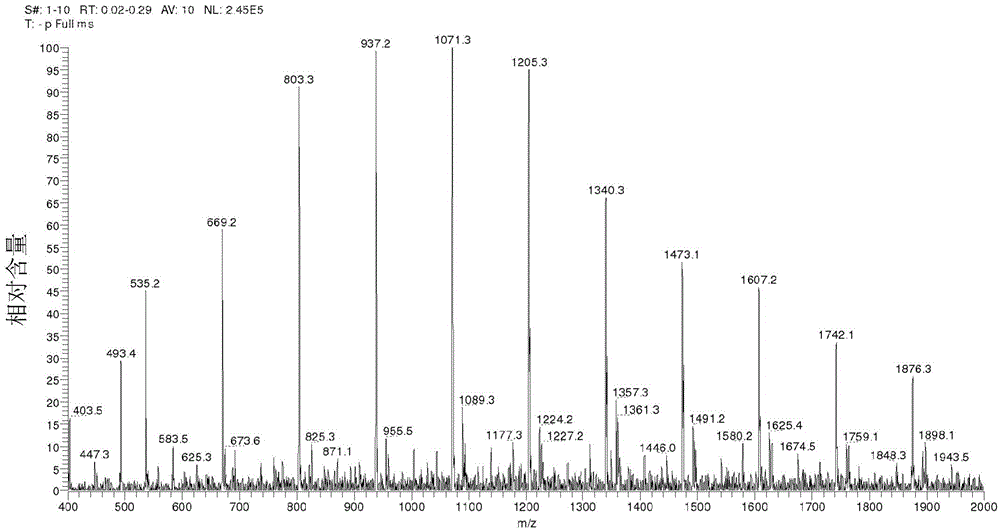
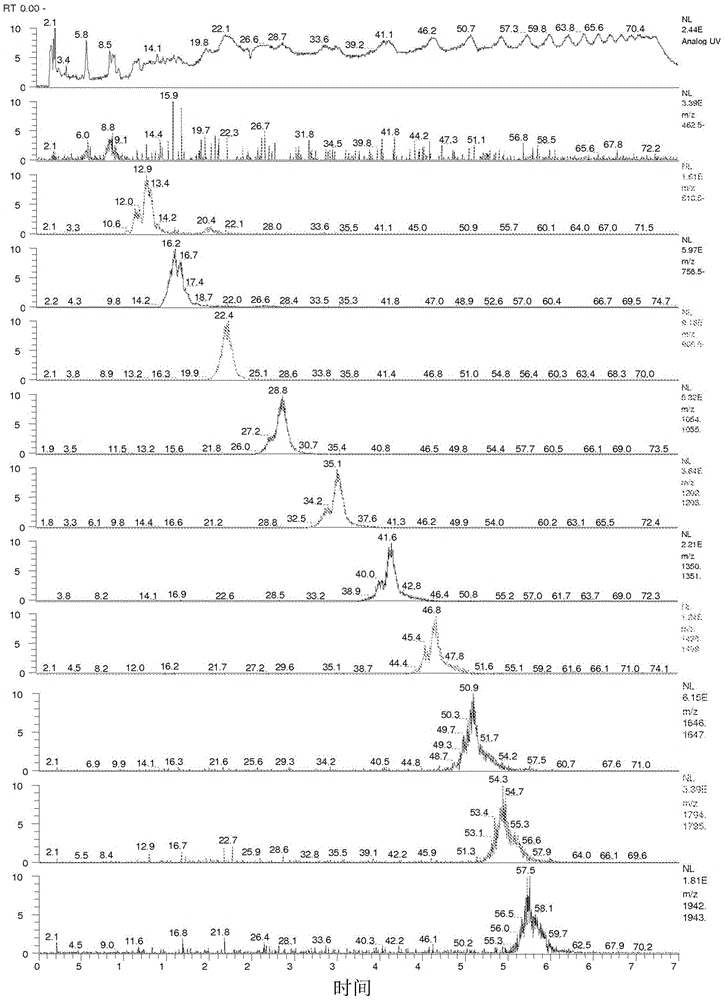
[0128] The structure of formula (3a) was further confirmed by electrospray ionization (ESI) mass spectrometry (MS). Several samples prepared according to the procedure of Example 1 were evaluated. Such as figure 1 As shown in , the ESI mass spectrum indicates that the product from Example 1 has a polymer composition of repeating units of 134 atomic mass units (amu), which corresponds to the molecular weight of mandelic acid minus water.
Embodiment 3
[0130] The second synthesis of the polymer of formula (3a) was achieved by performing polymerization at -30°C (±5°C). The concentrated sulfuric acid was cooled to -30°C. The temperature was maintained at -30°C (± 5 degrees) as mandelic acid was added in aliquots to the cooled and stirred mixture over a period of time (approximately 30 minutes for a 20 gram reaction). (The ratio of mandelic acid / concentrated sulfuric acid is 20 grams / 100ml). The reaction mixture was then stirred and maintained at -30°C for one hour, then the temperature was allowed to slowly rise to room temperature and stirred for a further 12 hours. The reaction mixture was then poured onto / into a mixture of ice and DI water (500 g / 200 ml). A pale pink precipitate formed and was then vacuum filtered, washed with water and then resuspended in 200 ml of DI water. The precipitate was collected by vacuum filtration and washed by repeated suspensions in DI water (200ml) followed by vacuum filtration until the f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com