Organic small molecular material based on spiro thioxanthene and organic photoelectric device using material as light emitting layer
A technology for optoelectronic devices and small molecules, applied in the fields of electric solid devices, electrical components, light-emitting materials, etc., can solve the problems of rarely reported organic small molecular materials, and achieve the effect of improving carrier transport characteristics and good solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
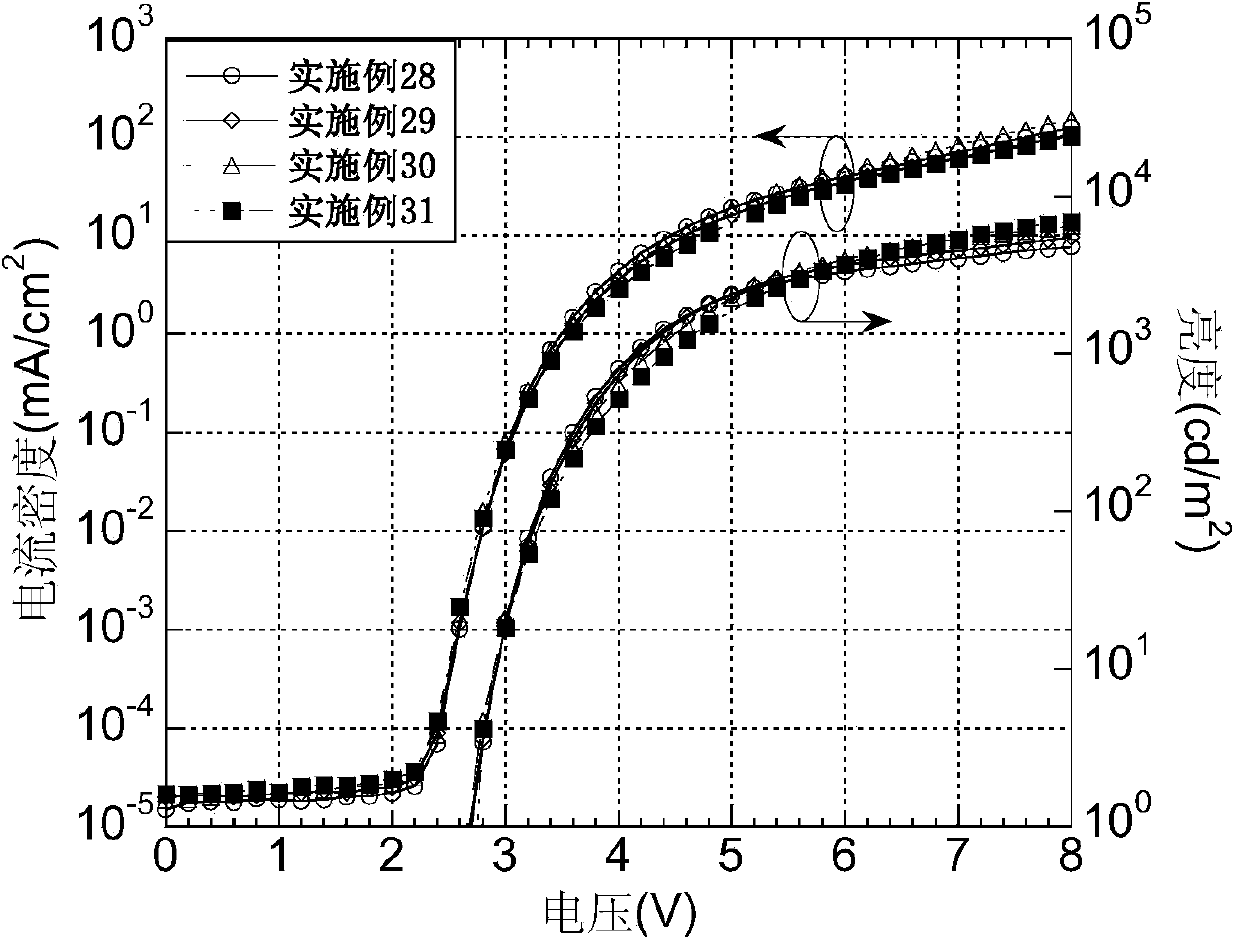
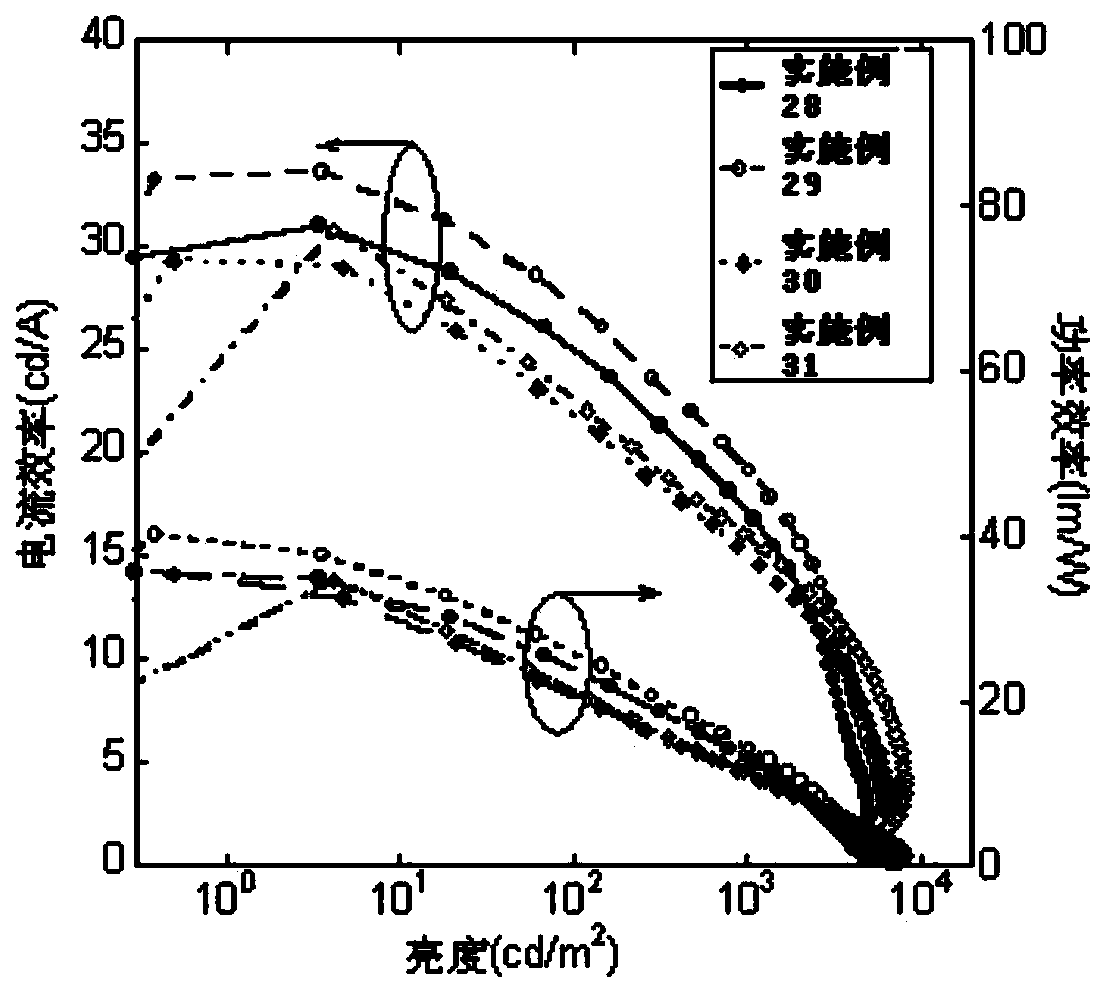
Examples
Embodiment 1
[0033] The preparation of present embodiment intermediate 1 and 2:
[0034] Synthesis of 2-bromodiphenylsulfide: o-bromoiodobenzene (50mmol, 14.3g), diphenyldisulfide (16.625mmol, 3.40g), Cu 2 S(1.194g), Fe(1.05g), K 2 CO 3 (8.625g) was dissolved in 125ml of DMSO, heated to 120°C overnight to obtain 2-bromodiphenylsulfide (3.84g), yield 93%. 1H NMR: 7.55-7.59 (m, 1H), 7.36-7.48 (m, 5H), 7.13-7.18 (m, 1H), 7.01-7.07 (m, 1H), 6.92-6.96 (m, 1H). The reaction equation is as follows:
[0035]
[0036] Synthesis of Intermediates 1 and 2: Dissolve 2-bromodiphenylsulfide (11mmol, 2.93g) in 60ml of anhydrous THF, cool to -78°C, add n-BuLi (14.36mmol) dropwise, and keep warm for 1 hour , Dissolve 4,4'-dibromobenzophenone (10mmol, 3.38g) in 30ml THF, add to react overnight, extract with dichloromethane, and pass through a silica gel column. White solid a (2.35g) was obtained with a yield of 53%; the white solid a (4.46mmol, 2.35g) was directly added to glacial acetic acid, heated...
Embodiment 2
[0039] In this embodiment, the preparation of a spirothioxanthene-based organic small molecule material having the structural formula P5:
[0040] Intermediate 2 (1.5mmol, 0.81g) obtained in Example 1, carbazole (3.3mmol, 0.57g), CuI (0.23g), K2CO3 (0.55g), C18O6 (0.1g), were dissolved in 1,3 -Dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone (DMPU), heated to 180°C overnight, extracted with dichloromethane, passed through a silica gel column to obtain 0.78g P5, yield 90% . 1H NMR: 8.33-8.34 (d, 2H), 8.14-8.16 (d, 4H), 7.65-7.67 (d, 4H), 7.56-7.58 (d, 4H), 5.49-5.51 (d, 4H), 7.41-7.44 (d, 4H), 7.29-7.31 (m, 6H), 7.17-7.18 (d, 4H).
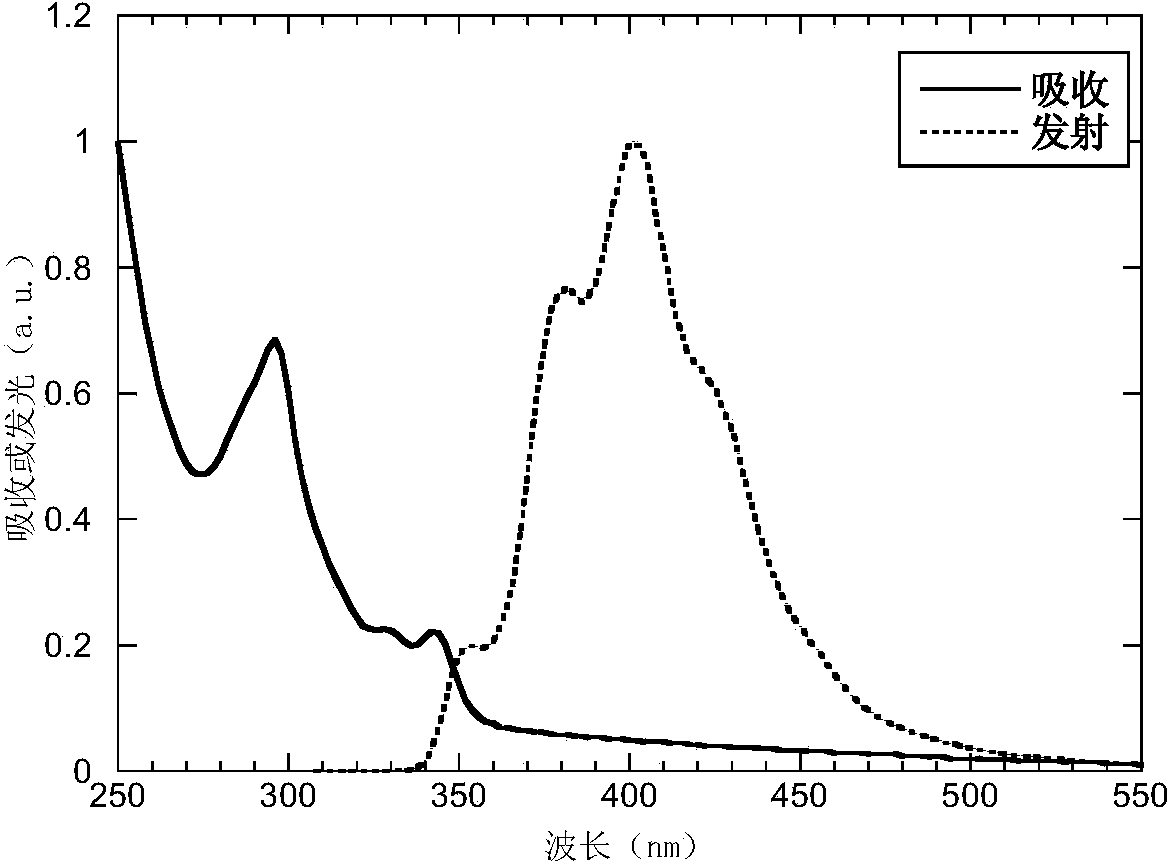
[0041] The absorption and emission spectrograms of the P5 of the present embodiment in the thin film state are as follows figure 1 As shown, from the absorption edge wavelength λ=358nm of the film, according to the formula optical band gap Eg opt =1240 / λ learned, Eg opt = 3.46eV.
Embodiment 3
[0043] In this embodiment, the preparation of a spirothioxanthene-based organic small molecule material having the structural formula P6:
[0044]Intermediate 1 (1.5mmol, 0.81g) obtained in Example 1, carbazole (3.3ml, 0.57g), CuI (0.23g), K2CO3 (0.55g), C18O6 (0.1g), were dissolved in DMPU, Heated at 180°C overnight, extracted with dichloromethane, and passed through the column to obtain 0.83g of P6. The yield was 82%. 1H NMR: 8.85-8.84 (d, 2H), 8.14-8.16 (d, 4H), 7.65-7.67 (d, 4H), 7.56-7.58 (d, 4H), 5.49-5.51 (d, 4H), 7.41-7.44 (d, 4H), 7.29-7.31 (m, 6H), 7.17-7.18 (d, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com