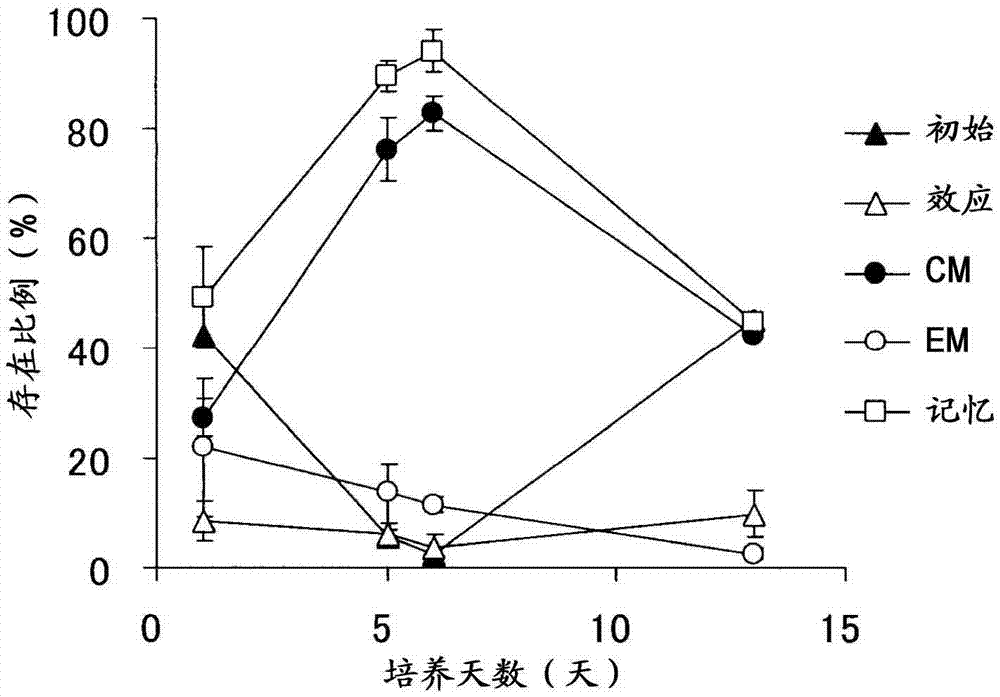
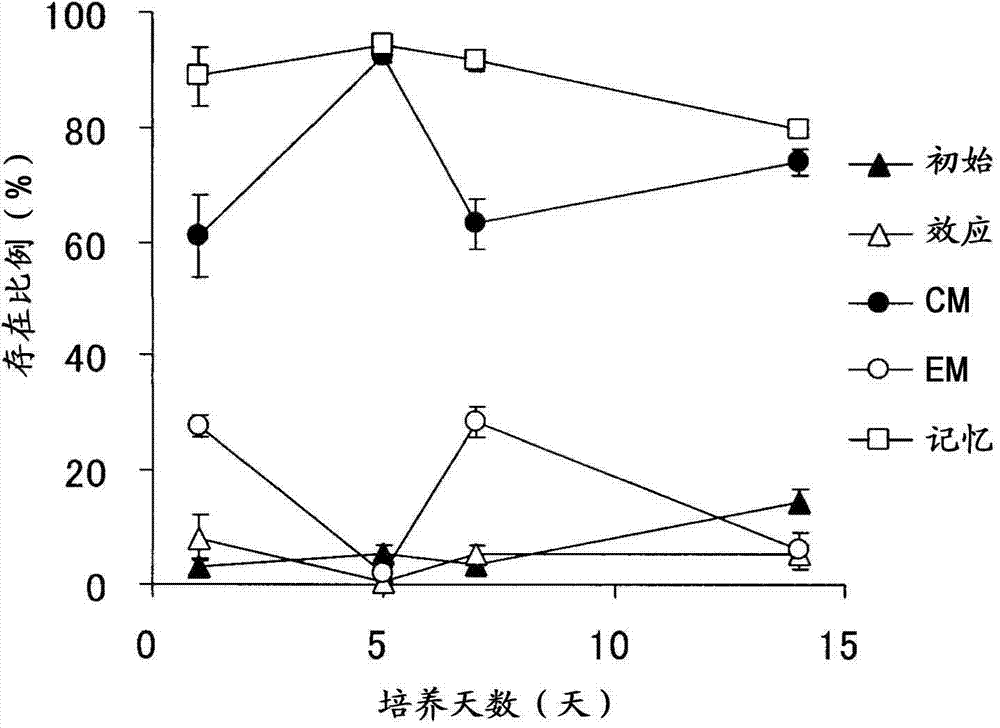
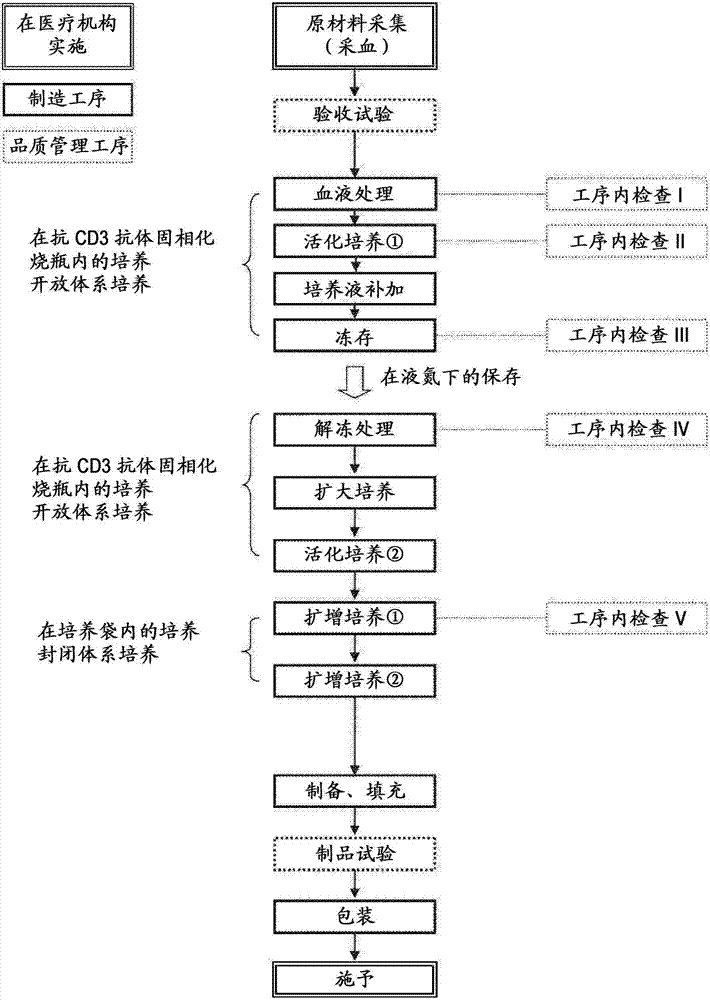
Method for producing lymphocyte cell group consisting mainly of memory t cells
A technology of lymphocytes and manufacturing methods, applied in the direction of animal cells, vertebrate cells, blood/immune system cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] [Activation and proliferation of lymphocytes]
[0072] (1) Preparation of peripheral blood lymphocytes
[0073]50 mL of peripheral blood was collected intravenously from 3 healthy people who had obtained consent by adding heparin. Each blood test sample was transferred to a 250 mL centrifugal sedimentation tube (BD Falcon; 352075), and 100 mL of washing solution (physiological saline supplemented with 0.1% human albumin: 500 mL of normal saline, manufacturer : Otsuka Pharmaceutical, 2.0mL 25% human blood albumin, manufacturer: Tanabe Mitsubishi Pharmaceutical), slowly mixed, diluted 3 times. The following operations including this one are performed under sterile conditions. For each test sample, 3-fold diluted blood was relayered in four 50 mL centrifugal sedimentation tubes (BD Falcon; 352070) filled with 15 mL of polysucrose (Ficoll) (manufactured by GE Healthcare Bioscience). The centrifugal precipitation tube was continuously centrifuged at 1800 rpm for 20 minute...
Embodiment 2
[0103] [Changes in Lymphocyte Surface Antigens During Activation Culture]
[0104] (1) Preparation and activation culture of lymphocytes
[0105] By the same procedure as in (1) of Example 1, peripheral blood lymphocytes were prepared from 3 healthy individuals who had obtained consent, and the number of cells was counted.
[0106] In the same manner as in (3) of Example 1, the prepared lymphocytes were centrifuged, and the cell density of the lymphocytes was adjusted to 6×10 with medium 1. 5 individual / mL. 50mL of this cell suspension was injected into the 225cm cell suspension made in (2) of Example 1. 2 In the OKT3 immobilized flask (manufactured by Sumitomo Bakelite Co., Ltd.: MS-2180R), medium 1 was used in the same manner as in Example 1 (3) in TE-HER type CO 2 In the incubator (manufactured by HIRASAWA Co., Ltd.), the temperature is 37.0±0.5°C, the humidity is 95.0±5.0%, CO 2 The concentration was 5.0±0.2% for cultivation. During the culture, suspended cells were c...
Embodiment 3
[0119] [Research on the density of frozen cells in the freezing process]
[0120] The preparation containing the T cell population obtained by the method of the present invention will be used for treatment, and the number of finally obtained lymphocytes is high to be effective. Therefore, the possibility that cryopreservation affects the number of cells per test tube after activation expansion culture was investigated.
[0121] (1) Study on frozen cell density-1 activation culture
[0122] By the same procedure as in (1) of Example 1, peripheral blood lymphocytes were prepared from 3 healthy individuals who had obtained consent, and the number of cells was counted.
[0123] In the same manner as in (3) of Example 1, the prepared lymphocytes were centrifuged, and the cell density of the lymphocytes was adjusted to 6×10 with medium 1. 5 individual / mL. 50mL of this cell suspension was injected into the 225cm cell suspension made in (2) of Example 1. 2 In the OKT3 solid-phase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com